Goodnight. Sleep Clean.
Notes from Dr. Norman Blumenstock:
Here is a great article about why sleep is SO important!!
Credit Eiko Ojala
SLEEP seems like a perfectly fine waste of time. Why would our bodies evolve to spend close to one-third of our lives completely out of it, when we could instead be doing something useful or exciting? Something that would, as an added bonus, be less likely to get us killed back when we were sleeping on the savanna?
“Sleep is such a dangerous thing to do, when you’re out in the wild,” Maiken Nedergaard, a Danish biologist who has been leading research into sleep function at the University of Rochester’s medical school, told me. “It has to have a basic evolutional function. Otherwise it would have been eliminated.”
We’ve known for some time that sleep is essential for forming and consolidating memories and that it plays a central role in the formation of new neuronal connections and the pruning of old ones. But that hardly seems enough to risk death-by-leopard-in-the-night. “If sleep was just to remember what you did yesterday, that wouldn’t be important enough,” Dr. Nedergaard explains.
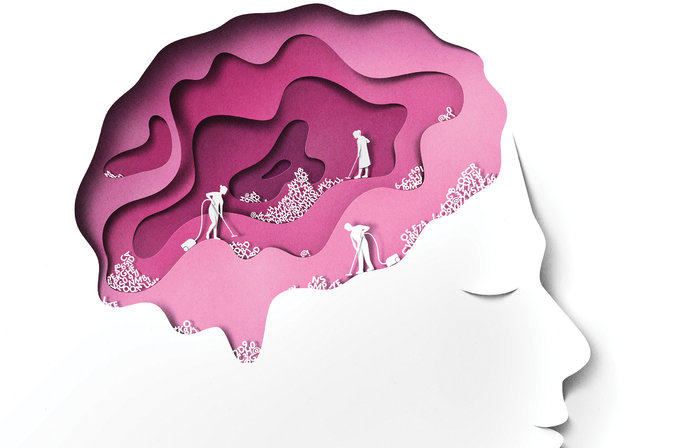
In a series of new studies, published this fall in the journal Science, the Nedergaard lab may at last be shedding light on just what it is that would be important enough. Sleep, it turns out, may play a crucial role in our brain’s physiological maintenance. As your body sleeps, your brain is quite actively playing the part of mental janitor: It’s clearing out all of the junk that has accumulated as a result of your daily thinking.
Recall what happens to your body during exercise. You start off full of energy, but soon enough your breathing turns uneven, your muscles tire, and your stamina runs its course. What’s happening internally is that your body isn’t able to deliver oxygen quickly enough to each muscle that needs it and instead creates needed energy anaerobically. And while that process allows you to keep on going, a side effect is the accumulation of toxic byproducts in your muscle cells. Those byproducts are cleared out by the body’s lymphatic system, allowing you to resume normal function without any permanent damage.
The lymphatic system serves as the body’s custodian: Whenever waste is formed, it sweeps it clean. The brain, however, is outside its reach — despite the fact that your brain uses up about 20 percent of your body’s energy. How, then, does its waste — like beta-amyloid, a protein associated with Alzheimer’s disease — get cleared? What happens to all the wrappers and leftovers that litter the room after any mental workout?
“Think about a fish tank,” says Dr. Nedergaard. “If you have a tank and no filter, the fish will eventually die. So, how do the brain cells get rid of their waste? Where is their filter?”
UNTIL a few years ago, the prevailing model was based on recycling: The brain got rid of its own waste, not only beta-amyloid but other metabolites, by breaking it down and recycling it at an individual cell level. When that process eventually failed, the buildup would result in age-related cognitive decline and diseases like Alzheimer’s. That “didn’t make sense” to Dr. Nedergaard, who says that “the brain is too busy to recycle” all of its energy. Instead, she proposed a brain equivalent of the lymphatic system, a network of channels that cleared out toxins with watery cerebrospinal fluid. She called it the glymphatic system, a nod to its dependence on glial cells (the supportive cells in the brain that work largely to maintain homeostasis and protect neurons) and its function as a sort of parallel lymphatic system.
She was hardly the first to think in those terms. “It had been proposed about one hundred years ago, but they didn’t have the tools to study it properly,” she says. Now, however, with advanced microscopes and dyeing techniques, her team discovered that the brain’s interstitial space — the fluid-filled area between tissue cells that takes up about 20 percent of the brain’s total volume — was mainly dedicated to physically removing the cells’ daily waste.
When members of Dr. Nedergaard’s team injected small fluorescent tracers into the cerebrospinal fluid of anesthetized mice, they found that the tracers quickly entered the brain — and, eventually, exited it — via specific, predictable routes.
The next step was to see how and when, exactly, the glymphatic system did its work. “We thought this cleaning process would require tremendous energy,” Dr. Nedergaard says. “And so we asked, maybe this is something we do when we’re sleeping, when the brain is really not processing information.”
In a series of new studies on mice, her team discovered exactly that: When the mouse brain is sleeping or under anesthesia, it’s busy cleaning out the waste that accumulated while it was awake.
In a mouse brain, the interstitial space takes up less room than it does in ours, approximately 14 percent of the total volume. Dr. Nedergaard found that when the mice slept, it swelled to over 20 percent. As a result, the cerebrospinal fluid could not only flow more freely but it could also reach further into the brain. In an awake brain, it would flow only along the brain’s surface. Indeed, the awake flow was a mere 5 percent of the sleep flow. In a sleeping brain, waste was being cleared two times faster. “We saw almost no inflow of cerebrospinal fluid into the brain when the mice were awake, but then when we anesthetized them, it started flowing. It’s such a big difference I kept being afraid something was wrong,” says Dr. Nedergaard.
Similar work in humans is still in the future. Dr. Nedergaard is currently awaiting board approval to begin the equivalent study in adult brains in collaboration with the anesthesiologist Helene Benveniste at Stony Brook University.
So far the glymphatic system has been identified as the neural housekeeper in baboons, dogs and goats. “If anything,” Dr. Nedergaard says, “it’s more needed in a bigger brain.”
MODERN society is increasingly ill equipped to provide our brains with the requisite cleaning time. The figures are stark. Some 80 percent of working adults suffer to some extent from sleep deprivation. According to the National Sleep Foundation, adults should sleep seven to nine hours. On average, we’re getting one to two hours less sleep a night than we did 50 to 100 years ago and 38 minutes less on weeknights than we did as little as 10 years ago. Between 50 and 70 million people in the United States suffer from some form of chronic sleep disorder. When our sleep is disturbed, whatever the cause, our cleaning system breaks down. At the University of Pennsylvania’s Center for Sleep and Circadian Neurobiology, Sigrid Veasey has been focusing on precisely how restless nights disturb the brain’s normal metabolism. What happens to our cognitive function when the trash piles up?
At the extreme end, the result could be the acceleration of neurodegenerative diseases like Alzheimer’s and Parkinson’s. While we don’t know whether sleep loss causes the disease, or the disease itself leads to sleep loss — what Dr. Veasey calls a “classic chicken-and-egg” problem — we do know that the two are closely connected. Along with the sleep disturbances that characterize neurodegenerative diseases, there is a buildup of the types of proteins that the glymphatic system normally clears out during regular sleep, like beta-amyloids and tau, both associated with Alzheimer’s and other types of dementia.
“To me,” says Dr. Veasey, “that’s the most compelling part of the Nedergaard research. That the clearance for these is dramatically reduced from prolonged wakefulness.” If we don’t sleep well, we may be allowing the very things that cause neural degeneration to pile up unchecked.
Even at the relatively more benign end — the all-nighter or the extra-stressful week when you caught only a few hours a night — sleep deprivation, as everyone who has experienced it knows, impedes our ability to concentrate, to pay attention to our environment and to analyze information creatively. “When we’re sleep-deprived, we can’t integrate or put together facts,” as Dr. Veasey puts it.
But there is a difference between the kind of fleeting sleep loss we sometimes experience and the chronic deprivation that comes from shift work, insomnia and the like. In one set of studies, soon to be published in The Journal of Neuroscience, the Veasey lab found that while our brains can recover quite readily from short-term sleep loss, chronic prolonged wakefulness and sleep disruption stresses the brain’s metabolism. The result is the degeneration of key neurons involved in alertness and proper cortical function and a buildup of proteins associated with aging and neural degeneration.
It’s like the difference between a snowstorm’s disrupting a single day of trash pickup and a prolonged strike. No longer quite as easy to fix, and even when the strike is over, there’s likely to be some stray debris floating around for quite some time yet. “Recovery from sleep loss is slower than we’d thought,” Dr. Veasey notes. “We used to think that after a bit of recovery sleep, you should be fine. But this work shows you’re not.”
If you put her own research together with the findings from the Nedergaard lab, Dr. Veasey says, it “very clearly shows that there’s impaired clearance in the awake brain. We’re really starting to realize that when we skip sleep, we may be doing irreparable damage to the brain, prematurely aging it or setting it up for heightened vulnerability to other insults.”