Positive airway pressure is the gold-standard for obstructive sleep apnea therapy, but an alternative therapy, when indicated, can literally be a lifesaver.
PHARMACOLOGICAL
Medical Weight Loss

Information source: Angela Fitch, MD, member of the board of trustees of the Obesity Medicine Association
How it works: The more a patient weighs, the more the airway collapses from the excess weight, leading to obstruction. Excess abdominal weight can play a role in lung volumes so can increase the tendency for airway collapse. Losing weight helps to reduce the fat mass around the neck and abdomen and therefore decreases the propensity for airway collapse/obstruction.
When to try it: In combination with CPAP or other airway-stabilizing methods.
Appropriate apnea hypopnea index (AHI): Appropriate for all obstructive sleep apnea (OSA) severities.
Other considerations: All patients with a body mass index (BMI) over 25 and OSA should be offered a comprehensive medical weight loss program to attain and sustain a 5% to 10% weight reduction to see if this resolves or improves their disease. The addition of medication to help with weight loss is indicated for patients with OSA and a BMI at or above 27. Patients with a BMI over 35 and OSA should be offered and evaluated for the appropriateness of metabolic surgery (see the “bariatric surgery” explanation later in this article).
The GLP-1 agonist liraglutide (brand name Saxenda, a prescription weight-loss medication) has been shown in studies to have an independent effect on OSA (improving OSA due to GLP-1 receptors that effect muscle control of the airway). So using Saxenda in patients with OSA may result in a double benefit—with the GLP-1 agonist’s effect on OSA directly and the improvement in weight loss from using an anti-obesity medication. More clinical trials are needed to show if this benefit is greater for this class of obesity medication versus others.
Many studies have shown that whatever lifestyle change helps the patient lose weight is what is best for them. The Mediterranean lifestyle, low-carbohydrate lifestyle, and meal-replacement programs combined with increased physical activity, treatment of OSA, and medications for obesity treatment are successful at sustaining long-term 5% to 10% weight loss and improving OSA severity.
Patients who should NOT try it: Patients who are normal weight and have OSA or for some other reason are not needing to lose weight.
New developments in 2017: None
Concerns & responses:
Concern: Medical weight loss is not covered by most payors.
Fitch: Medicare covers intensive behavior therapy for obesity and over time the hope is other payors will as well.
Concern: For many patients, there isn’t a significant reduction in AHI.
Response: A study by Blackman A, et al showed a reduction in AHI of 12 events per hour.
More information: Blackman A, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). Epub 2016 March 23.
DEVICES
Oral Appliances
 Information source: Harold A. Smith, DDS, president of the American Academy of Dental Sleep Medicine (AADSM)
Information source: Harold A. Smith, DDS, president of the American Academy of Dental Sleep Medicine (AADSM)
How it works: Oral appliances are mandibular advancement devices (MADs) that protrude and help stabilize the jaw in order to maintain a patent upper airway during sleep.
When to try it: First-line therapy: Oral appliances are a first-line treatment for primary snoring, after a physician has ruled out OSA.
After CPAP failure: A clinical practice guideline published jointly in 2015 by the American Academy of Sleep Medicine (AASM) and American Academy of Dental Sleep Medicine (AADSM) recommends that sleep physicians consider prescription of oral appliance therapy for adult patients with OSA who are intolerant of CPAP therapy or prefer an alternate therapy.
CPAP is superior to oral appliance therapy in improving AHI and lowering both the arousal index and the oxygen desaturation index (ODI). However, the efficacy gap between CPAP and oral appliance therapy may be closed when adherence is taken into consideration. The literature suggests the treatment adherence rate for oral appliance therapy is greater than for CPAP therapy. This clinical reality suggests that the overall therapeutic effectiveness of oral appliances is comparable with CPAP.
Combination therapy: For patients who struggle with CPAP due to a high air pressure setting, combination therapy—which involves using an oral appliance in conjunction with CPAP—can improve treatment adherence by lowering the air pressure setting required to normalize breathing.
Appropriate AHI: Most effective for mild (5<AHI<15) to moderate (15<AHI<30) OSA. Although oral appliance therapy can reduce obstructive respiratory events in adult patients with severe OSA (AHI of 30 or more), it is less likely than CPAP to normalize severe OSA to an AHI of 5 or below. However, for patients with severe OSA who do not adhere to CPAP, an oral appliance with partial effectiveness that is worn all night long is a much better alternative than no treatment.
Other considerations: Patients who are most responsive to oral appliance therapy generally tend to be those with a lower AHI (less than 30) and BMI.
Patients who should NOT try it: Oral appliance therapy typically does not prove to be effective for patients with morbid obesity, defined as having a BMI of 40 or more. Several physical features can also prevent oral appliance therapy from being an effective treatment for adults, including:
- Steep mandibular plane
- Long neck
- Poor dentition, such as loose teeth or not having enough teeth (the patient should have at least 6-10 teeth in each arch)
- Acute temporomandibular joint dysfunction (TMD) or disc displacement resulting in a limited ability to open the mouth
New developments in 2017: Researchers are continuing to evaluate potential predictors of success for oral appliance therapy while developing tools and processes that may improve appliance selection and titration. For example, an abstract presented at the 2017 AADSM Annual Meeting and published in the Journal of Dental Sleep Medicine evaluated the use of a fully digital clinical workflow for oral appliance therapy (poster #021: Utilizing a fully digital clinical workflow for oral appliance therapy with an auto-titrating mandibular positioner (AMP): a feasibility study.).
Concerns & responses:
Concern: Dentists will require the patient to pay out-of-pocket for the device due to mixed information about insurance coverage.
Smith: Medicare and most US medical insurers cover oral appliance therapy from a dentist as they would a CPAP machine from a physician, if the patient has been diagnosed with OSA and prescribed oral appliance therapy by a physician.
Concern: There’s no way of knowing if oral appliance therapy will work on patients before they try it.
Smith: It is important for sleep physicians to collaborate with a dentist who can conduct a thorough intra-oral examination to assess candidacy for an oral appliance. The dentist will evaluate the patient’s teeth, jaw, and airway, determine the protrusive range with a measuring device, and review the data of the sleep study in order to help determine the chance of success.
Concern: Most oral appliances don’t objectively monitor compliance.
Smith: The research investigating objective compliance measurement for oral appliance therapy continues to grow. The most well-known study by Vanderveken, published in Thorax, demonstrated the feasibility of an embedded micro-sensor to provide objective measurement and calculate the mean disease alleviation. However, it should be noted that the study also found no significant differences between objective and self-reported oral appliance compliance. Therefore, until objective compliance monitoring becomes standard, self-reports from patients can provide useful clinical insight.
Concern: Oral appliances have side effects.
Smith: Oral appliances have side effects and so does CPAP; in fact, literature shows both modalities of treatment move teeth. The body of relevant research continues to expand. The winner of the 2017 AADSM Student Research Excellence Award, Mona Hamoda, BDS, MSc, MHSc, was recognized for leading the longest evaluation to date of oral appliance therapy side effects, with an average follow-up period of 12.6 years (poster #001: Long-term side effects of sleep apnea treatment with oral appliances.). The study indicates that long-term treatment of OSA with oral appliance therapy requires ongoing follow-up and reassessment by both a qualified dentist and qualified physician. The study analyzed baseline and follow-up lateral cephalograms of 62 patients treated with oral appliance therapy for OSA. Results show significant and progressive dental changes with prolonged oral appliance use, including reduction in overjet and overbite, but there were no clinically significant skeletal changes.
More information: Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013 Apr 15;187(8):879-87.
Night Shift
 Information source: Daniel J. Levendowski, president, Advanced Brain Monitoring
Information source: Daniel J. Levendowski, president, Advanced Brain Monitoring
How it works: Worn on the back of the neck, Night Shift (by Advanced Brain Monitoring) begins to vibrate when users begin to sleep on their back and slowly increases in intensity until a position change occurs.
When to try it: First line therapy for positional OSA; in combination with oral appliance therapy, surgery, or Provent, or with CPAP to lower CPAP pressure.
Appropriate AHI: Night Shift was cleared by the FDA for positional mild, moderate, or severe OSA when nonsupine AHI<20.
Other considerations: While positional therapy can reduce the overall AHI to the nonsupine severity, its effect on snoring is more variable. For patients with an elevated supine apnea index, overall snoring may increase with positional therapy because supine apneas are replaced with snoring. Patients who are interested in tracking their sleep quality will appreciate the device’s capability to measure nightly changes in sleep time, awakenings, snoring, and position.
Patients who should NOT try it: Those with acute neck, shoulder, or back pain, those with cardiac arrhythmia corrected with an artificial pacemaker, or those who have skin sensitivity or an open wound around the neck.
New developments in 2017: Using the Positional Sleep Assessment System software, Night Shift can be used in combination with the Nonin WristOx to monitor outcomes.
Concerns & responses:
Concern: Some studies show poor long-term compliance with positional therapy.
Levendowski: In 135 patients across 15 to 52 weeks, overall Night Shift compliance was 70% and overall regular use (>-4 h/night over 70% of nights) was 88%.
Concern: Night Shift is reactive. It doesn’t keep the patient off his back to start.
Levendowski: Night Shift is programmable to deliver supine avoidance feedback immediately, or most commonly with a 15-minute delay to allow the user an opportunity to fall asleep.
Concern: Night Shift will wake my patient due to the vibration, thereby still interrupting his sleep.
Levendowski: Patient attempt to sleep supine on average 5 to 7 times/night. So positional feedback causes approximately 1 arousal per hour, substantially less than the number of arousals per hour eliminated as a resulting benefit of positional therapy.
Concern: Patients do not like to wear something around their neck.
Levendowski: From a treatment efficacy standpoint, the back of the neck is the optimal location for the measurement and delivery of positional therapy. It directly reflects when gravity is contributing to the collapse of the airway. The nape of the neck has the greatest number of active sensory receptors as well as relatively low body fat, factors that impact the ability to perceive vibration. This means less time is spent supine because patients respond faster at a lower level of vibration, as compared to other regions of the body.
More information: Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10(8):863-71.
Provent Sleep Apnea Therapy
 Information source: John Guinipero, national sales manager, Provent Sleep Therapy LLC
Information source: John Guinipero, national sales manager, Provent Sleep Therapy LLC
How it works: Provent uses the power of the user’s breathing to create expiratory positive airway pressure (EPAP) that helps keep the airway open.
When to try it: Can be used as a first line therapy; after refusal or failure of CPAP; and is also used by some CPAP users as a travel therapy.
Appropriate AHI: Appropriate for all OSA severities.
Other considerations: Any patient with mild, moderate, or severe sleep apnea who has difficulty tolerating CPAP or who prefers a different option for sleep apnea therapy.
Patients who should NOT try it: Patients with severe breathing disorders including hypercapnia respiratory failure, respiratory muscle weakness, bulls lung disease, bypassed upper airway, pneumothorax, pneumomediastinum, etc. Those with severe heart disease (including heart failure). Those with pathologically low blood pressure. Those with an acute upper respiratory (including nasal, sinus, or middle ear) inflammation or infection, or perforation of the ear drum.
New developments in 2017: Provent launched updated English instructions for use (IFU) as well as new international IFUs in 8 languages (French, Spanish, Italian, Dutch, German, Portuguese, Simplified Chinese, and Traditional Chinese).
Provent’s updated Starter Kit lets users step up to Provent therapy over the first several nights with lower resistance patches, enhanced users instructions, and educational materials.
In 2017, more than 6,000,000 nights of Provent therapy have been prescribed to date.
Concerns & responses:
Concern: Provent can’t objectively monitor compliance.
Guinipero: The non-electrical nature of Provent’s convenient disposable devices means that compliance can’t be monitored directly. Provent does however offer equipment so sleep studies can be conducted to determine the effectiveness of therapy in patients.
Concern: There’s no way of knowing if Provent will work on patients before they try it.
Guinipero: Provent does offer equipment so sleep studies can be conducted to determine the effectiveness of therapy in patients.
Concern: Most payors won’t cover Provent.
Guinipero: We are working to promote Provent Therapy across the country to patients, doctors, and insurance companies. It is already covered by some insurance companies: Kaiser Permanente, Veterans Affairs (the VA), and some Blue Cross Plans. Some insurance companies will also reimburse for Provent when it is submitted under HCPCS code E1399. In addition, because Provent is a prescription item, it can be purchased with flexible spending accounts (FSAs and Health Savings Accounts/HSAs).
Concern: Some patients take longer to acclimate than what is offered in the Provent Starter Kit.
Guinipero: We are consulting with physicians to determine whether custom starter packs would be a more optimal solution for their patients.
More information: Riaz M, Certal V, Nigam G, Abdullatif J, Zaghi S, Clete Kushida CA, Camacho M. Nasal expiratory positive airway pressure devices (Provent) for OSA: a systematic review and meta-analysis. Sleep Disord. 2015: 734798.
Winx
 Information source: David L. Jones, chief business officer, ApniCure
Information source: David L. Jones, chief business officer, ApniCure
How it works: Winx delivers a light vacuum through a soft flexible mouthpiece, which helps keep the airway open during sleep by keeping the tongue and soft palate from falling back and blocking the airway.
When to try it: Those who fail or refuse CPAP; those who are “grudgingly-compliant” with CPAP may benefit from switching; those who have tried dental appliances and/or surgeries without success can also try to see if Winx works for them.
Appropriate AHI: Appropriate for all OSA severities.
Other considerations: Users must be able to breathe comfortably through their nose with their mouth closed and have most of their teeth (so the mouthpiece stays in place). Winx can be purchased online, no office visits are required, and the mouthpiece sizing is done through the mail. There is a 60-day 100% money-back guarantee.
Patients who should NOT try it: The Winx system should not be used to treat central sleep apnea or by anyone who has a severe respiratory disorder, such as severe lung disease, pneumothorax, etc; has loose teeth or advanced periodontal disease; is under the age of 18.
New developments in 2017: Winx now sells direct to consumers. In select states, the company can connect patients with board-certified sleep medicine physicians who will consult via telemedicine to see if a patient’s condition merits a prescription for Winx or another treatment.
Concerns & responses:
Concern: Most payors won’t cover Winx.
Jones: Like many new therapies, Winx is not covered by Medicare or private insurance (but it is covered by the VA). The company offers payment options, including one for about $54/month. If you have an FSA through your employer or a HSA you can use those “pre-tax” funds toward the cost of your Winx system.
Concern: There’s no way of knowing if Winx will work on patients before they try it.
Jones: For any therapy to treat your obstructive sleep apnea, it must meet 2 criteria: 1) it must be effective for you when you use it, and 2) you must consistently use it. Because of this, no sleep apnea therapy treats a large majority of OSA patients.
Even though CPAP is highly effective in the sleep lab, half of CPAP users can’t tolerate it and become non-compliant within the first year—leaving their sleep apnea essentially untreated. Dental appliances and surgery also don’t work for everyone and there is typically no money back if these therapies don’t work for you.
86% of Winx users experienced improvement in their sleep apnea and 59% of Winx users were either much improved or very much improved, according to assessments by their physicians in recent clinical trials.
If you can tolerate using CPAP through the night and use it most nights, then continue using it. If you aren’t using CPAP, however, and are looking for a sleep apnea treatment, try Winx. It requires minimal effort to try and, if you decide it’s not right for you, you can return it for a full refund.
Concern: Patients won’t want to empty the saliva canister in the morning.
Jones: We don’t hear about that topic very much from our customers. It takes less than a minute to rinse out the reservoir and rinse/clean the mouthpiece. It’s not that different from cleaning a mouthguard or rinsing off a toothbrush after use.
More information: Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med. 2013;14:830-7.
IN-OFFICE PROCEDURES
Pillar Procedure
 Information source: Craig Schwimmer, MD, MPH, FACS, chief medical officer, Pillar Palatal LLC
Information source: Craig Schwimmer, MD, MPH, FACS, chief medical officer, Pillar Palatal LLC
How it works: The Pillar Procedure is a minimally invasive palatal stiffening technique, usually performed in-office using local anesthesia. Permanent suture is inserted into the soft palate, inducing scar formation. This stiffens and stabilizes the palate, reducing airway vibration and collapse.
When to try it: First line therapy for patients who refuse or fail CPAP or who simply prefer another treatment approach.
Appropriate AHI: Mild to moderate OSA.
Other considerations: The Pillar Procedure should be considered in any patient for whom there appears to be a significant palatal component to the snoring and/or sleep apnea. Studies (including two meta-analyses) show not only a meaningful impact on snoring, but also significant reductions in both AHI and RDI (respiratory disturbance index). But because palatal stiffening is often therapeutically necessary but not sufficient, the most relevant clinical question for these patients may be: Which other minimally invasive procedures should be performed in conjunction with the Pillar Procedure in order to maximize clinical impact? For example, if the patient’s airway is compromised by both palatal flutter and nasal airway obstruction, combining a Pillar Procedure with a minimally invasive nasal procedure is likely to optimize the clinical outcome. If the palate and tongue base appear to be the most relevant anatomic sites, combining a Pillar Procedure with either oral appliance therapy or tongue base radio frequency ablation (RFA) may be most effective.
Patients who should NOT try it: Patients with a known allergy to the implant material or those whose soft palate is not long enough to accommodate the implants.
New developments in 2017: The significance of the dose-response relationship between the number of implants placed and clinical improvement has now been widely demonstrated and accepted, and as a result, most physicians routinely use 5 Pillar implants per patient. Also, the Pillar Procedure is now most commonly used not as a standalone procedure, but as part of a more comprehensive multi-level minimally invasive approach. Combining the Pillar Procedure with other minimally invasive procedures (such as turbinate reduction, tongue base RFA, oral appliance therapy, etc) is now routinely done in order to optimize results across a wider array of patients, while avoiding the cost, risk, and morbidity of traditional surgical techniques.
Concerns & responses:
Concern: There is a risk of extrusion of the implant.
Schwimmer: The Pillar Procedure has been performed in over 75,000 patients. The implant material has been used for over 50 years. Not one significant Pillar Procedure complication has ever been reported to the FDA. Extrusion has been reported in fewer than 2% of all cases. Implant extrusion has never been reported to result in any significant morbidity, and the risk of extrusion is most appropriately seen as merely a potential nuisance.
Concern: AHI doesn’t significantly improve for most patients.
Schwimmer: A meta-analysis of studies using 3 implants per patient showed an average 26% reduction in disease severity. Additionally, most experienced practitioners report even better results with additional implants, and additionally improved outcomes when the Pillar Procedure is combined with other minimally invasive treatments (such as turbinate reduction, nasal alar improvement, tongue base RFA, or oral appliance therapy).
Concern: The Pillar Procedure alone may not adequately treat a patient’s OSA.
Schwimmer: This is true. But if only perfect treatments deserve consideration, then we have nothing to offer any of our OSA patients. Most human beings are not compliant with CPAP. Oral appliance compliance is generally reported to be less than 70%, and among patients who wear one, the expected reduction in disease severity is approximately 50%. Despite the significant cost, risk, and morbidity of surgical treatment, there remains to be described a perfectly effective surgical procedure. So what all treatment options really represent are sets of compromises. Cost, risk, morbidity, need for compliance, and expected efficacy all should weigh into our treatment making decisions. Using the Pillar Procedure as the foundation of a multi-level, minimally invasive, office-based treatment approach represents a compelling set of compromises for a surprisingly high percentage of patients.
Concern: Except within the VA system, the Pillar Procedure is not covered by insurance, which may make it too expensive for patients.
Schwimmer: The cost of a Pillar Procedure is often less than the cost of a custom oral appliance. Additionally, because patients now typically have high deductibles, it is actually often much less expensive for a patient to undergo a “non-covered procedure” in the office, than to undergo a “covered procedure” in the operating room. Lastly, because the Pillar Procedure allows patients to immediately return to normal activities, the indirect economic costs of surgery are avoided.
More information: Neruntarat C. Long-term results of palatal implants for obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2011 Jul;268(7):1077-80. doi: 10.1007/s00405-011-1511-4. Epub 2011 Feb 5.
Somnoplasty
 Information source: Information provided in 2014 from an Olympus America Inc associate product manager, but we did not receive a response to our emailed or phone requests in 2017 for an update
Information source: Information provided in 2014 from an Olympus America Inc associate product manager, but we did not receive a response to our emailed or phone requests in 2017 for an update
How it works: Somnoplasty, also known as temperature-controlled radiofrequency (TCRF), is a minimally invasive surgical technology that uses radiofrequency current to reduce tissue volume in a precise, targeted manner. (Somnoplasty device by Olympus pictured.)
When to try it: After refusal or failure of CPAP; first line therapy for patients who choose TCRF when offered all options.
Appropriate AHI: Mild to moderate OSA.
Other considerations: None provided.
Patients who should NOT try it: There are no known absolute contraindications to the use of RF surgery. The use of somnoplasty probes and G3 RF workstation is contraindicated when—in the judgment of the physician—electrosurgical procedures are contrary to the best interest of the patient.
New developments in 2017: None provided.
Concerns & responses:
Concern: There is a risk of bleeding and infection.
Concern: It reduces snoring but not sleep apnea.
(Responses not provided.)
More information: Powell NB, Riley RW, Troell RJ, Blumen MB, Guilleminault C. Radiofrequency volumetric reduction of the tongue. A porcine pilot study for the treatment of obstructive sleep apnea syndrome. Chest. 1997;111(5):1348-55.
SURGERIES
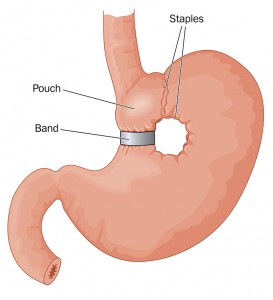
Bariatric Surgery
 Information source: Information provided in 2014 by John Morton, MD, MPH, FACS, FASMBS, chief, Bariatric and Minimally Invasive Surgery, Stanford School of Medicine (At deadline in 2017, Morton was on paternity leave and the American Society for Metabolic and Bariatric Surgery did not respond to an emailed request for a spokesperson.)
Information source: Information provided in 2014 by John Morton, MD, MPH, FACS, FASMBS, chief, Bariatric and Minimally Invasive Surgery, Stanford School of Medicine (At deadline in 2017, Morton was on paternity leave and the American Society for Metabolic and Bariatric Surgery did not respond to an emailed request for a spokesperson.)
How it works: Gastric banding, sleeve gastrectomy, and gastric bypass surgery lead to significant weight loss, including lessening the buildup of fat tissue in the upper thorax and neck. Studies have shown that weight loss may result in improvements to OSA.
When to try it: First line therapy for patients with BMIs greater than 40.
Appropriate AHI: 15 and above.
Other considerations: BMI and AHI are the main considerations.
Patients who should NOT try it: Patients with severe pulmonary hypertension need a more extensive plan to be prepared for surgery.
New developments in 2017: None provided.
Concerns & responses:
Concern: Most payors won’t cover bariatric surgery.
Morton: Bariatric surgery is routinely covered by major insurers. If it’s not covered, you should make an inquiry as to why it is not. It’s safe and effective for patients in need.
Concern: AHI may not significantly improve.
Morton: There are many studies that have demonstrated AHI reductions. Additionally, bariatric surgery and subsequent weight loss provide better treatment options for patients if they are in need of further treatment. For instance, it can improve CPAP adherence.
More information: Aguiar IC, et al. Obstructive sleep apnea and pulmonary function in patients with severe obesity before and after bariatric surgery: a randomized clinical trial. Multidiscip Respir Med. 2014;9(1):43.
Uvulopalatopharyngoplasty (UPPP) with or without Tonsillectomy
 Information source: Kathleen L. Yaremchuk, MD, MSA, department Chair, Otolaryngology Head and Neck Surgery, Henry Ford Hospital
Information source: Kathleen L. Yaremchuk, MD, MSA, department Chair, Otolaryngology Head and Neck Surgery, Henry Ford Hospital
How it works: The most common type of oropharyngeal surgery for OSA, UPPP enlarges the retropalatal upper airway by excising a portion of the soft palate and uvula with trimming and reorientation of the tonsillar pillars. The tonsils, if present, may be excised as well. In adults with enlarged tonsils, tonsillectomy alone has been shown to be effective in eliminating obstructive sleep apnea in adults.
When to try it: After refusal or failure of CPAP.
Appropriate AHI: N/A (AHI is not a success predictor.)
Other considerations: Anatomy is the main predictor of success. Favorable anatomy for UPPP with or without tonsillectomy includes large tonsils and favorable tongue position (Malampatti 1 or 2 classification).
Patients who should NOT try it: Some argue that patients with a BMI of 40 and above should opt for bariatric surgery over oropharyngeal surgery. But Yaremchuk has had success with UPPP with or without tonsillectomy in patients with BMIs in this range and so characterizes high BMI as a “relative” contraindication.
New developments in 2017: More and more surgeons are opting to perform a drug-induced sleep endoscopy (DISE) prior to oropharyngeal surgery in order to find out exactly where the blockage is and help determine the responders versus the non-responders. Many studies have shown that it may impact the procedure the surgeon chooses to perform but outcomes regarding surgical success have not changed significantly.
Concerns & Responses:
Concern: There is a risk of infection.
Yaremchuk: Infection is very uncommon—it’s probably the least likely problem you’ll experience with this surgery.
Concern: AHI may not significantly improve.
Yaremchuk: AHI is not the only indicator of significant OSA improvement. Success may be better determined by another marker such as ODI or an inflammatory indicator that is more indicative of sleep apnea’s risks. Hypersomnolence may be relieved with surgical intervention for OSA. Also, surgery is not a magic bullet for sleep apnea. But when patients reject or can’t tolerate CPAP, surgery is a viable option. A huge problem is when patients are unwilling or unable to tolerate CPAP, they feel there are no other alternative therapies.
*Additional surgery options:
Surgical intervention needs to be tailored to the patient’s level of obstruction in the upper airway, Yaremchuk says. Options include:
- transoral robotic surgery (TORS) excises all or part of the lingual tonsils or resects a portion of the base of tongue.
- maxillary mandibular advancement (MMA) is a procedure in which the upper and lower jaws are surgically moved anteriorly to enlarge the airway.
- Inspire Upper Airway Stimulation or hypoglossal nerve implant is a surgical procedure that does not alter the upper airway anatomy but is physiologic in that an upper-airway stimulation system (see separate explanation for Inspire Upper Airway Stimulation later in this article).
More information: Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002 Jul;127(1):13-21.
Smith MM, Peterson E, Yaremchuk KL. The role of tonsillectomy in adults with tonsillar hypertrophy and obstructive sleep apnea. Otolaryngol Head Neck Surg. 2017 Aug;157(2):331-5. doi: 10.1177/0194599817698671. Epub 2017 Mar 28.
Inspire Upper Airway Stimulation (UAS)
 Information source: Inspire Medical Systems
Information source: Inspire Medical Systems
How it works: Inspire therapy is an implanted system that senses breathing patterns and delivers mild stimulation to key airway muscles keeping the airway open during sleep.
When to try it: After CPAP refusal or failure.
Appropriate AHI: 15 to 65.
Other considerations: In addition to the AHI consideration, patients might be candidates for Inspire therapy if they are unable to use or get consistent benefit from CPAP, are not significantly overweight, and are over the age of 22. An Inspire therapy-trained doctor will also evaluate the patient’s overall health status and perform a physical examination of the airway to determine if Inspire therapy might be a suitable CPAP alternative.
Patients who should NOT try it: Those with central + mixed apneas >25% of the total AHI; any anatomical finding that would compromise the performance of upper airway stimulation, such as the presence of complete concentric collapse of the soft palate
New developments in 2017: In May 2017, the FDA approved the next generation Inspire device. The Inspire 3028 is 40% smaller and 18% thinner than the original device and includes MRI conditional labeling.
Also in 2017, the 5-year STAR trial results were published that demonstrated the significant objective and subjective patient outcomes seen at 12, 24, and 48 months were sustained at 60 months.
Concerns & responses:
Concern: There is a risk of infection.
Inspire: All surgeries carry a small risk of infection. In contrast to other surgical options to treat sleep apnea, Inspire therapy does not require removing or permanently altering a patient’s facial or airway anatomy. As such, the procedure is less invasive and should result in a shorter recovery time. It also does not require a mask or oral appliance. The serious adverse event rate in the STAR trial was <2%.
Concern: It’s uncertain what the long-term side effects are of continuously stimulating the hypoglossal nerve.
Inspire: After 60 months of follow-up, there have been no reported long-term side effects from stimulating the hypoglossal nerve.
Concern: I have to refer my patient out to another physician for this therapy.
Inspire: Although you will need to refer your patients to a different doctor for the DISE and surgical procedure, you, as a sleep physician, are responsible for ongoing patient management to include pre-, post-, and long-term care of your patient.
Concern: I am uncertain whether Inspire will be covered by third-party payors in my area.
Inspire: Inspire therapy is being reviewed and approved by insurance providers on a case-by-case basis. Inspire Medical Systems works with physicians to share commonly billed codes and to teach how to properly submit the initial coverage request, any subsequent appeals, and titration-related sleep studies.
More information: Strollo PJ, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139-49.





