Are you or your partner CPAP intolerant? Stop the CPAP torture and discover the No Mask options available with Dr. Blumenstock. Oral appliances offer a No Mask solution to CPAP treatment. No Mask, No Straps, No Hoses and No Head Gear! Now you can sleep comfortably and wake rested!
Oral Appliance therapy involves the selection, design, fitting and use of a specially designed oral appliance that, when worn during sleep, maintains an opened, unobstructed airway in the throat. Oral appliances that treat snoring and obstructive sleep apnea are devices worn in the mouth, similar to orthodontic retainers or sports mouth-guards. They have several advantages over other forms of therapy. Oral appliances are comfortable and easy to wear and care for. They are small and convenient, making them easy to carry with you when you travel. Treatment with oral appliances is reversible and non-invasive.

Narval CC
- Computer-aided design (CAD) allows for a high degree of customization to fit the complex dental anatomy of each patient
- Computer-aided manufacturing (CAM) using selective laser sintering of a biocompatible polymer material guarantees consistent, industrial-strength manufacturing
- Use of polyamide for a slim, lightweight device that is highly resilient and durable
Narval CC


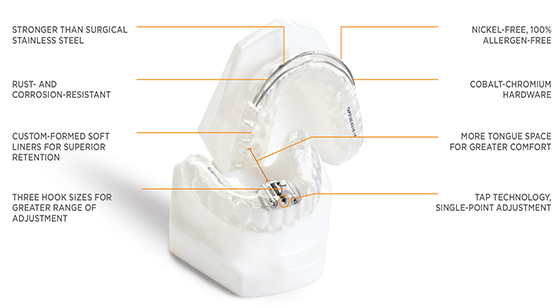
dreamTAP
 Intended to reduce or alleviate nighttime snoring and obstructive sleep apnea (OSA), the dreamTAP appliance affords greater tongue space with no obstruction. The nickel-free, cobalt chromium hardware is on the exterior — and the dreamTAP’s single point of adjustment prevents the uneven bilateral adjustment which can cause irregular bite and jaw discomfort.
Intended to reduce or alleviate nighttime snoring and obstructive sleep apnea (OSA), the dreamTAP appliance affords greater tongue space with no obstruction. The nickel-free, cobalt chromium hardware is on the exterior — and the dreamTAP’s single point of adjustment prevents the uneven bilateral adjustment which can cause irregular bite and jaw discomfort.
Incorporate the Braebon Compliance Monitor (DentiTrac™) with the dreamTAP oral appliance to measure clinical treatment.
How it works
- Using dreamTAP with compliance recorder -powered by DentiTrac™-, patient compliance can now be objectively measured when using a dreamTAP oral device
- The compliance recorder registers the length of time the device is worn and the patient’s supine or non-supine head position
- Using the base station, clinicians can upload patient data into HIPAA-secure Cloud
The benefits of dreamTAP with compliance recording
- Uniquely Compliant. 96% of patients would continue using their dreamTAP device
- Highly Effective. 15+ independent clinical studies demonstrate dreamTAP efficacy
- Uncompromised Accuracy. Measure hours worn & head position and delivers daily summaries
- HIPAA Secure – Cloud Based. Your patient & practice records are secure & accessible from anywhere
- Technical Specifications. Includes a thermal sensor, accelerometer and a 3 year device warranty
dreamTAP


Micr02® Sleep and Snore Device

- Absolutely no metal in the device
- A smoother, more comfortable surface
- Up to 22% more strength in every direction
- 30% less overall volume
- 33% less mouth opening required
- Better airway protection
- 3.6X fewer monomers and residual methyl methacrylate for better taste, lowered toxicity, and decreased possibility of allergic reaction
- Less or no tooth movement for improved comfort and accuracy
- The longest warranty available – up to three years
Incorporate the Braebon Compliance Monitor (DentiTrac™) with the Micr02® oral appliance to measure clinical treatment.
How it works
- Using Micr02® with compliance recorder -powered by DentiTrac™-, patient compliance can now be objectively measured when using a Micr02® oral device
- The compliance recorder registers the length of time the device is worn and the patient’s supine or non-supine head position
- Using the base station, clinicians can upload patient data into HIPAA-secure Cloud
The benefits of Micr02® with compliance recording
- Uniquely Compliant. 96% of patients would continue using their Micr02® device
- Highly Effective. 15+ independent clinical studies demonstrate Micr02® efficacy
- Uncompromised Accuracy. Measure hours worn & head position and delivers daily summaries
- HIPAA Secure – Cloud Based. Your patient & practice records are secure & accessible from anywhere
- Technical Specifications. Includes a thermal sensor, accelerometer and a 3 year device warranty
Micr02® Sleep and Snore Device


SomnoDent Herbst Advance™
SomnoDent Herbst Advance™…Innovation in Calibration
 The SomnoDent Herbst Advance is the first and only intraoral device featuring a visual calibration indicator for the treatment of OSA, giving you and your patients greater control over OSA therapy.
The SomnoDent Herbst Advance is the first and only intraoral device featuring a visual calibration indicator for the treatment of OSA, giving you and your patients greater control over OSA therapy.
Herbst Advance™ Benefits
- High level of patient comfort and satisfaction
- E0486 Medicare approved
- Reduced chair time due to minimal adjustments needed
- Proprietary SMH BFlex lining that does not attract odors or delaminate
Supported by years of medical research - 3 year product warranty against manufacturing defects for Medicare patients; 2 years for Non-Medicare patients FDA 510(k) cleared, Class II Medical Devices
- All devices manufactured under 21 CFR 820 and ISO 13485 standards
Advanced Comfort: Especially when made with proprietary SMH BFlex, the SomnoDent Herbst Advance features a hinge mechanism that is more comfortable in the patient’s mouth; making it easier to remain compliant
Enhanced Control: Herbst Advance can be easily adjusted using our visual indicator, giving you and your patients total control of their treatment
Less Resets: With an 8mm range of calibration, less device resets will be needed; saving you chair time and ensuring patients’ continuous therapeutic efficacy
SomnoDent Herbst Advance™


SomnoDent® with Compliance Recorder

SomnoDent® with Compliance Recorder: Meet the first and only oral device with compliance recording technology.
SomnoMed is proud to introduce Somnodent with compliance recorder – a definitive breakthrough merging oral device therapy with reliable compliance results you can depend on, only available through SomnoMed. SomnoDent with compliance recorder is designed to specially address the need for objective compliance measurement when using oral devices to treat obstructive sleep apnea.
How it works
- Using SomnoDent with compliance recorder -powered by DentiTrac™-, patient compliance can now be objectively measured when using a SomnoDent oral device
- The compliance recorder registers the length of time the device is worn and the patient’s supine or non-supine head position
- Using the base station, clinicians can upload patient data into HIPAA-secure Cloud
The benefits of SomnoDent with compliance recording
- Uniquely Compliant. 96% of patients would continue using their SomnoDent device
- Highly Effective. 15+ independent clinical studies demonstrate SomnoDent efficacy
- Uncompromised Accuracy. Measure hours worn & head position and delivers daily summaries
- HIPAA Secure – Cloud Based. Your patient & practice records are secure & accessible from anywhere
- Technical Specifications. Includes a thermal sensor, accelerometer and a 3 year device warranty
Incorporate SomnoDent with CR into your practice with confidence
- All SomnoDent devices can be fitted with a compliance recorder to measure clinical treatment, especially among CPAP intolerant patients
- In the US, SomnoDent devices will be the first and only dorsal fin oral devices with compliance recorder powered by DentiTrac and in countries outside of the US, all SomnoDent devices will be the only oral devices with compliance recorder powered by DentiTrac.
- Many physicians are interested in their patients’ compliance and now that can be recorded by the compliance recorder and compliance is objectively measured
- Many employers and payers offer Sleep Wellness programs to their employees, and realize cost savings and productivity gains for the company through improved health of their employees
SomnoDent® with Compliance Recorder

OASYS Hinge Appliance
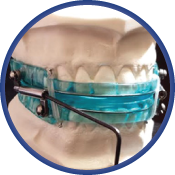 Dream Systems’ OASYS Hinge Appliance, with Nasal Dilators and with Nasal Dilators and Lingual Tongue Buttons.
Dream Systems’ OASYS Hinge Appliance, with Nasal Dilators and with Nasal Dilators and Lingual Tongue Buttons.
Features include:
- The T-10 Hinge is a smaller profile hinge, which allows for more flexible placement, even in shorter arch cases.
- It is also a telescope designed hinge that does not come a part with full extension.
- It provides 10 mm of advancement, in comparison to the current 5 mm hinge.
- It is designed for intra-oral activation, in comparison of side activation out of the mouth.
- One half turn (12:00 to 6:00 clockwise) = 1/4 mm using a 1.27 mm hex wrench; one full revolution (12:00 to 12:00 clockwise) = 1/2 mm; and 2 revolutions clockwise equaling 1 mm advancement. (16 Clicks = 1 mm Scheu Hinge).
- The T-10 Hinge has etched mm marks on the hinge for identification of advancement, compared to the Scheu hinge which does not.
- There is a Nylock coating in the hinge chamber to prevent backing off of the screw, plus a welded stop to prevent the screw from ever coming out of the chamber.
- The Hex Wrench will activate the hinge screws for advancement and also is used for tightening or loosening the hex screws on the pivots.
- The OASYS Hinge can also include the benefits of the OASYS Oral Nasal Airway System, with the available options of the Nasal Dilators and Lingual Tongue Lifters.
OASYS Hinge Appliance
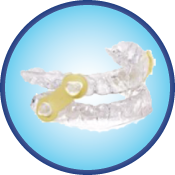

Elastic Mandibular Advancement Appliance (EMA)
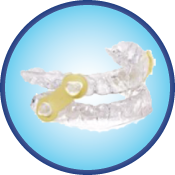 The EMA – Custom appliance is a simple, patient-friendly oral appliance created for noninvasive treatment of snoring and OSA. The primary treatment mechanism of opening the bite and gently moving the mandible forward is achieved with the use of interchangeable elastic straps that offer varying degrees of mandibular advancement. The flexibility of these elastic straps provides unsurpassed lateral movement and overall TMJ comfort. The 2 mm thick pressure formed bases offer orthodontic retention (resulting in no tooth movement) and maximum anterior tongue space because there are no projections in the palate.
The EMA – Custom appliance is a simple, patient-friendly oral appliance created for noninvasive treatment of snoring and OSA. The primary treatment mechanism of opening the bite and gently moving the mandible forward is achieved with the use of interchangeable elastic straps that offer varying degrees of mandibular advancement. The flexibility of these elastic straps provides unsurpassed lateral movement and overall TMJ comfort. The 2 mm thick pressure formed bases offer orthodontic retention (resulting in no tooth movement) and maximum anterior tongue space because there are no projections in the palate.
Elastic Mandibular Advancement Appliance (EMA)
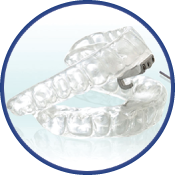

Thornton Adjustable Positioner
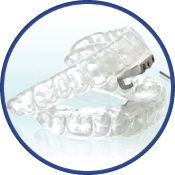
Most Effective for Severe Apnea
The TAP 1 diagnosis appliance is the only appliance that can be titrated in the sleep lab while the patient is asleep. The Thornton Adjustable Positioner uses the principle of cardiopulmonary resuscitation to keep the airway open in order to help patients maintain proper breathing techniques. Oxygen is allowed to flow adequately into the airway with the help of a device that holds the lower jaw forward to prevent collapse of the airway and eliminate instances of breathing cessation. With improved breathing, patients are able to get a good night’s rest and give their partners a chance to sleep with decreased snoring. The TAP device is comfortable and adjustable to fit patients’ unique size and shape mouths.
Thornton Adjustable Positioner


The SUAD™ Device
 The SUAD™ Device is a premium dental sleep appliance developed for the treatment of snoring and obstructive sleep apnea. It is an effective, comfortable, and durable alternative to CPAP therapy or corrective surgery. By simply wearing The SUAD™ Device while sleeping, your lower jaw (mandible) will be moved forward into a comfortable position, allowing relaxation of the tissues at the back of your throat and ensuring the base of your tongue does not collapse and block your airway, giving you a safe and soundless sleep.
The SUAD™ Device is a premium dental sleep appliance developed for the treatment of snoring and obstructive sleep apnea. It is an effective, comfortable, and durable alternative to CPAP therapy or corrective surgery. By simply wearing The SUAD™ Device while sleeping, your lower jaw (mandible) will be moved forward into a comfortable position, allowing relaxation of the tissues at the back of your throat and ensuring the base of your tongue does not collapse and block your airway, giving you a safe and soundless sleep.
The SUAD™ Device
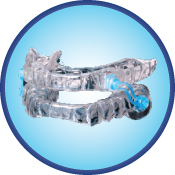

Silent Nite SL
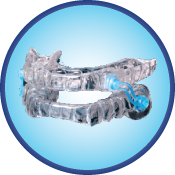 Silent Nite SL positions the lower jaw forward using special S-shaped connectors that are attached to upper and lower trays. These trays are comprised of a soft inner layer with a hard outer layer that is durable and BPA-free. The new, improved connectors are stronger than those found on the original Silent Nite and are easily interchangeable by the patient. This intraoral appliance is indicated for patients with a minimum of eight teeth per jaw and a body mass index (BMI) of 30 or less.
Silent Nite SL positions the lower jaw forward using special S-shaped connectors that are attached to upper and lower trays. These trays are comprised of a soft inner layer with a hard outer layer that is durable and BPA-free. The new, improved connectors are stronger than those found on the original Silent Nite and are easily interchangeable by the patient. This intraoral appliance is indicated for patients with a minimum of eight teeth per jaw and a body mass index (BMI) of 30 or less.
Silent Nite SL
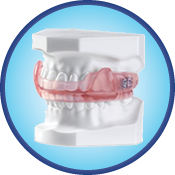

Dorsal Appliance
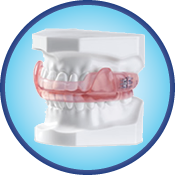 Originally designed as a nighttime TMD splint, the Dorsal Appliance has evolved into one of the most popular choices for snoring and obstructive sleep apnea. The two piece construction allows for patient comfort and lateral jaw movement. The Dorsal fins on the mandibular appliance interface with inclines built into the buccal of the upper appliance to dictate a specific mandibular position. The appliance is traditionally fabricated with adjustable screws in the maxillary appliance to allow for further mandibular advancement. This appliance can be fabricated in a variety of materials including acrylic, dual laminate or thermal splint material.
Originally designed as a nighttime TMD splint, the Dorsal Appliance has evolved into one of the most popular choices for snoring and obstructive sleep apnea. The two piece construction allows for patient comfort and lateral jaw movement. The Dorsal fins on the mandibular appliance interface with inclines built into the buccal of the upper appliance to dictate a specific mandibular position. The appliance is traditionally fabricated with adjustable screws in the maxillary appliance to allow for further mandibular advancement. This appliance can be fabricated in a variety of materials including acrylic, dual laminate or thermal splint material.
Dorsal Appliance


The Oasys Appliance
 The Oasys Appliance has a built-in nasal dilation so it works in a similar manner to Breathe Right Strips. The Oasys Oral/Nasal Airway System is the first dental device to be reviewed by both the dental and ENT divisions of the FDA. It has been approved as a dental device for treatment of snoring and sleep apnea through mandibular repositioning and also as a nasal dilator for reduction of nasal resistance and improved nasal breathing.
The Oasys Appliance has a built-in nasal dilation so it works in a similar manner to Breathe Right Strips. The Oasys Oral/Nasal Airway System is the first dental device to be reviewed by both the dental and ENT divisions of the FDA. It has been approved as a dental device for treatment of snoring and sleep apnea through mandibular repositioning and also as a nasal dilator for reduction of nasal resistance and improved nasal breathing.
The Oasys Appliance
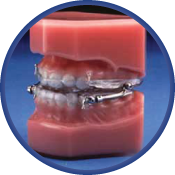

The Herbst Appliance
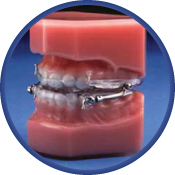 The Herbst appliance has been proven to be effective on chronic snoring and mild to moderate obstructive sleep apnea sufferers. This appliance allows patients to move laterally and vertically without disengaging the appliance. Also, if it is determined that the initial position does not provide the anticipated relief of the condition, the mandible can easily be moved forward by two options of adjustability. The Herbst is traditional hardware with limited range of adjustment. The Herbst Telescopic version allows greater flexibility of adjustment. The more robust SAUD version is made for the heavy grinder who might break other appliances.
The Herbst appliance has been proven to be effective on chronic snoring and mild to moderate obstructive sleep apnea sufferers. This appliance allows patients to move laterally and vertically without disengaging the appliance. Also, if it is determined that the initial position does not provide the anticipated relief of the condition, the mandible can easily be moved forward by two options of adjustability. The Herbst is traditional hardware with limited range of adjustment. The Herbst Telescopic version allows greater flexibility of adjustment. The more robust SAUD version is made for the heavy grinder who might break other appliances.
The Herbst Appliance
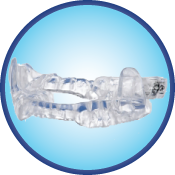

ClearDream®
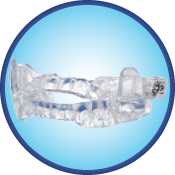 ClearDream® is an oral appliance for the treatment of snoring and mild to moderate sleep apnea in adults.
ClearDream® is an oral appliance for the treatment of snoring and mild to moderate sleep apnea in adults.
HOW IT WORKS
ClearDream is a dorsal appliance, as it uses ‘fins’ on the lower arch to advance the mandible. The ClearDream maintains an open airway using titratable posterior hardware in the upper arch to adjust the degree the lower jaw is moved forward.
The ClearDream is made with Keller’s clinically unbreakable Clear 450® material. We also employ a wax/design/inject manufacturing process that allows the lower arch with fin to be fabricated in one piece, greatly reducing the likelihood of fin breakage.
ADVANTAGES
- Durable:Made with Keller’s clinically unbreakable Clear 450®
- Design: Scalloped/contoured lingual surfaces for patient comfort and additional tongue space
- Retention: No ball clasps required – the combination of the design and Clear 450® material achieves sufficient retention
INDICATIONS
ClearDream is intended to reduce night time snoring and mild to moderate Obstructive Sleep Apnea (OSA) in adults.
CONTRAINDICATIONS
This device is contraindicated for patients who:
- have central sleep apnea
- have severe respiratory disorders
- have loose teeth or advanced periodontal disease
- are under 18 years of age
- missing 1st and 2nd molars in any quadrant
ClearDream®
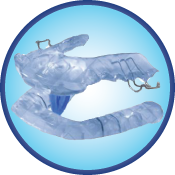

Sleep-Well™ Appliance
 Keeps the airway open by holding the jaw and tongue in a comfortable forward position while triggering a slight tightening of the soft tissues and muscles of the upper airway, especially the soft palate. FDA-cleared for Snoring and OSA, the Sleep Well Appliance is ideal for use in bruxing cases. It allows up to 9mm of AP advancement and maintains constant protrusiveness via the patented Protrusive Element (PE) inserts. Simple, non-threatening design and easy-to-clean, easy-to-adjust convenience yield high patient compliance.
Keeps the airway open by holding the jaw and tongue in a comfortable forward position while triggering a slight tightening of the soft tissues and muscles of the upper airway, especially the soft palate. FDA-cleared for Snoring and OSA, the Sleep Well Appliance is ideal for use in bruxing cases. It allows up to 9mm of AP advancement and maintains constant protrusiveness via the patented Protrusive Element (PE) inserts. Simple, non-threatening design and easy-to-clean, easy-to-adjust convenience yield high patient compliance.
Sleep-Well™ Appliance
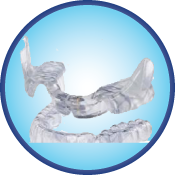

Aligner Sleep Appliance (ASA)™
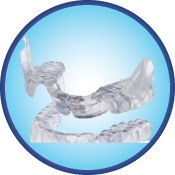 Dentist-prescribed for aligner/Invisalign® therapy, the Aligner Sleep Appliance (ASA)™ is worn with the Invisialign® appliance during sleep to support the lower jaw in a mild forward position, while reducing nighttime snoring and mild-to-moderate Obstructive Sleep Apnea in adults.
Dentist-prescribed for aligner/Invisalign® therapy, the Aligner Sleep Appliance (ASA)™ is worn with the Invisialign® appliance during sleep to support the lower jaw in a mild forward position, while reducing nighttime snoring and mild-to-moderate Obstructive Sleep Apnea in adults.
- Two piece construction ensures patient comfort
- Dual-side expansion screws activate to gradually move the jaw forward
- Custom design and full adjustability
Aligner Sleep Appliance (ASA)™


SML CLEAR SLEEP™ APPLIANCE
 The SML CLEAR SLEEP™ APPLIANCE reduces nighttime snoring and mild-to-moderate Obstructive Sleep Apnea in adults by moving the mandible forward in adjustable increments and opening the airway for easier, unrestricted breathing.
The SML CLEAR SLEEP™ APPLIANCE reduces nighttime snoring and mild-to-moderate Obstructive Sleep Apnea in adults by moving the mandible forward in adjustable increments and opening the airway for easier, unrestricted breathing.
Customized to meet the needs of each individual patient, the SML CLEAR SLEEP™ APPLIANCE is comprised of two lightweight, low volume trays that are comfortable, retentive, and BPA-free. Lots of tongue space, too.
- Patented stabilizing pins enhance strength and durability
- Interchangeable connectors (attached to upper and lower trays) can be changed to move the mandible forward in 1mm increments.
- Up to 7mm of advancement (based upon protrusive bite)
- Greater lateral movement
- Easy-to-change connector rods
- Discluding element may be added
- Available in vacuum form or acrylic material
SML CLEAR SLEEP™ APPLIANCE
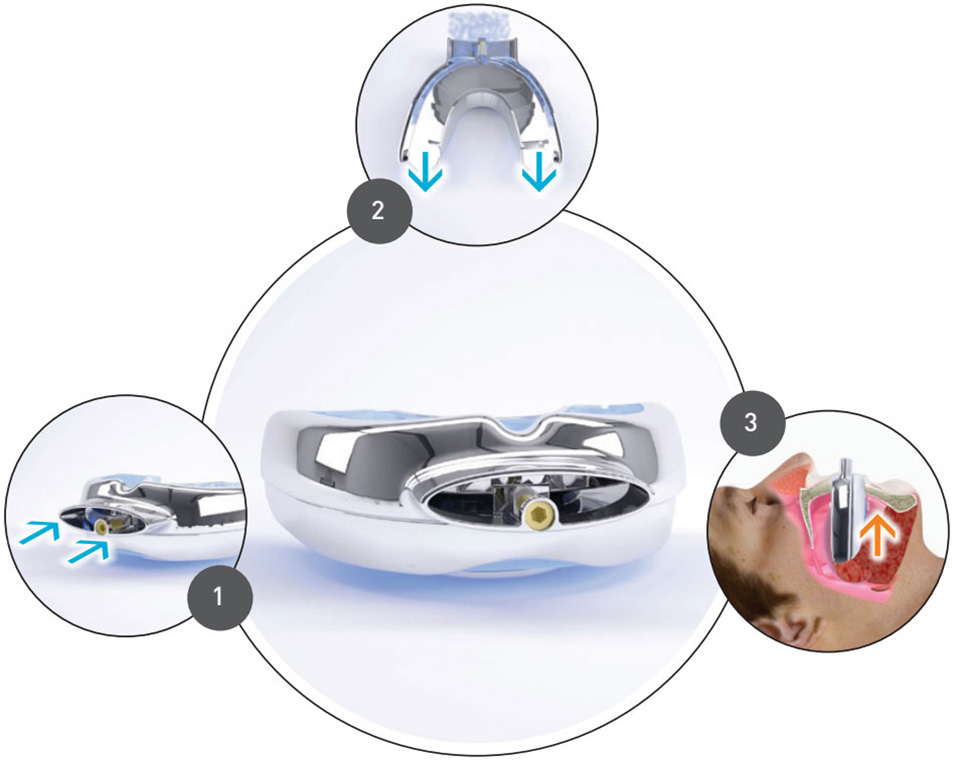

O2Vent™ Oral Appliance
The customized O2Vent™ devices may be particularly beneficial for the many people with nasal obstructions, those that experience soft palate collapse or who are habitual mouth breathers.
How the O2Vent™ works
1 – Air is drawn into the front of the device
2 – Air passes through to the back of the device
3 – The device advances the lower jaw/mandible, bringing the tongue forward and opening the airway
For a more detailed explanation of how the unique design allows for breathing through the device, bypassing obstruction in the nose which can contribute to snoring and sleep apnea, simply watch this video.
O2Vent™ Oral Appliance


O2Vent Optima

The US Food and Drug Administration (FDA) has cleared Oventus Medical’s O2Vent Optima, a customized 3D-printed nylon oral appliance, for the treatment of obstructive sleep apnea (OSA).
The O2Vent Optima is a mandibular advancement device that includes a separate airway to add stability to the upper airway for OSA patients throughout the night, the company announced this week in a press release.
O2Vent Optima
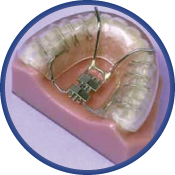

The Klearway Appliance
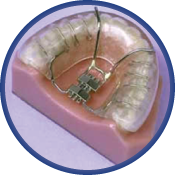 Klearway is a fully-adjustable oral appliance used for the treatment of snoring and mild to moderate Obstructive Sleep Apnea. Fabricated of thermoactive acrylic resin, Klearway becomes pliable for easy insertion and confirms securely to the dentition for an excellent fit while significantly decreasing soft tissue and tooth discomfort. Small increments (0.25mm) of forward lower jaw advancement are initiated by the patient under the direction of a dentist and this helps avoid rapid jaw movements that can cause significant patient discomfort. The appliance does not encroach on tongue space. Once warmed under hot water and inserted, the acrylic resin hardens as it cools to body temperature and firmly affixes itself to both arches. Lateral and vertical jaw movement is permitted which enables the patient to yawn, swallow, and drink water without dislodging the appliance.
Klearway is a fully-adjustable oral appliance used for the treatment of snoring and mild to moderate Obstructive Sleep Apnea. Fabricated of thermoactive acrylic resin, Klearway becomes pliable for easy insertion and confirms securely to the dentition for an excellent fit while significantly decreasing soft tissue and tooth discomfort. Small increments (0.25mm) of forward lower jaw advancement are initiated by the patient under the direction of a dentist and this helps avoid rapid jaw movements that can cause significant patient discomfort. The appliance does not encroach on tongue space. Once warmed under hot water and inserted, the acrylic resin hardens as it cools to body temperature and firmly affixes itself to both arches. Lateral and vertical jaw movement is permitted which enables the patient to yawn, swallow, and drink water without dislodging the appliance.
The Klearway Appliance
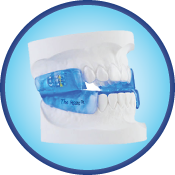

The Moses Appliance
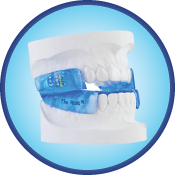 This new, innovative device adds another dimension to oral-appliance therapy. Stimulating protrusive tongue reflexes and an open anterior design advances the tongue and enlarges the cross-sectional area of the airway. Combined with mandibular advancement, it becomes the most effective appliance available. Two-part construction allows patients to talk, drink, open wide and close their lips, making this the most comfortable appliance available.
This new, innovative device adds another dimension to oral-appliance therapy. Stimulating protrusive tongue reflexes and an open anterior design advances the tongue and enlarges the cross-sectional area of the airway. Combined with mandibular advancement, it becomes the most effective appliance available. Two-part construction allows patients to talk, drink, open wide and close their lips, making this the most comfortable appliance available.
The Moses Appliance


Tongue Retaining Device (TRD)
 The TRD is lab-constructed flexible polyvinyl material adapted to the general contours of the teeth and dental arches. It does not depend on teeth for retention. Rather, the tongue is held forward by the negative pressure created in the vacuum bulb on the front of the appliance.
The TRD is lab-constructed flexible polyvinyl material adapted to the general contours of the teeth and dental arches. It does not depend on teeth for retention. Rather, the tongue is held forward by the negative pressure created in the vacuum bulb on the front of the appliance.
Tongue Retaining Device (TRD)


ProSomnus® `{`CA`}` Sleep and Snore Device
Introducing ProSomnus [CA], the only precision Continuous Advancement OAT device. ProSomnus [CA] utilizes proprietary technologies to resolve the archform asymmetries, manufacturing variances, and device design limitations of traditional OAT devices. ProSomnus [CA] is engineered to reduce side effects, enhance clinical efficiencies, and deliver predictable results.

ProSomnus® [CA] Sleep and Snore Device


Panthera DSAD, D-SAD Digital Sleep Apnea Device
DSAD, D-SAD
Digital Sleep Apnea Device (D-SAD or DSAD) from Panthera Dental. The D-SAD is an oral appliance that is designed and formed using CAD computer aided design. The device is made of nylon, so it is very light weight and smooth to the touch. The formation process of these mouthpieces is not unlike the 3D printing process.
This sleep apnea oral appliance is made of an upper maxillary retainer and a lower mandibular retainer. These two pieces of the DSAD are connected by a connecting or retaining rod. These connectors come in different sizes which allow for the adjust of the oral appliance.
The D-SAD by Panthera Dental sleep apnea dental device is similar in appearance and design as other CAD CAM oral therapy.
It is FDA approved for the treatment of sleep apnea and snoring. Generally, sleep apnea oral appliance therapy is recommended for the treatment of mild-moderate sleep apnea. However, it is also indicated for those that cannot or will not use CPAP therapy.
Because the Pathera Digital Sleep Apnea Device is computer designed and made of strong light

Panthera DSAD, D-SAD Digital Sleep Apnea Device


DentiTrac®
The patent-pending DentiTrac® is a miniature micro-recorder designed to measure patient treatment compliance using Braebon’s extensive world-leading experience in sensors and recorders.
Compliance measurement has been used in sleep therapy CPAP devices for years with numerous benefits.
Now DentiTrac® extends this capability into traditional oral appliance therapy.
An Amazing Fit
The smallest, most powerful compliance tracking recorder fits seamlessly into almost any oral appliance.
Accuracy without compromise
Measuring a wide range of metrics, from temperature
to three dimensional rotation, enables sophisticated
algorithms to determine accurate wearing times. This
power comes without compromising the micro-
recorder’s long lifespan.
Drop in. Data out.
With the DentiTrac base station and a web-enabled PC, compliance data is easily extracted from the micro-recorder and sent to the cloud.
Learn more here: https://www.braebon.com/products/dentitrac/