FDA Clears O2Vent Optima Oral Device for Obstructive Sleep Apnea
Published on September 3, 2019
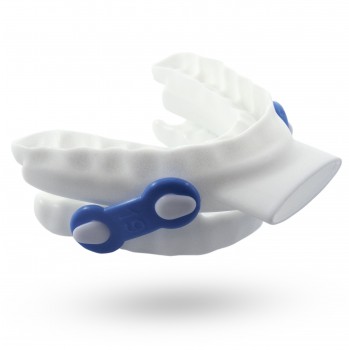
The US Food and Drug Administration (FDA) has cleared Oventus Medical’s O2Vent Optima, a customized 3D-printed nylon oral appliance, for the treatment of obstructive sleep apnea (OSA).
The O2Vent Optima is a mandibular advancement device that includes a separate airway to add stability to the upper airway for OSA patients throughout the night, the company announced this week in a press release.
According to the release, the FDA clearance follows the announcement in July that Oventus had signed its first material contract with an American sleep medicine group to adopt the Company’s O2Vent treatment platform for the treatment of OSA across 8 clinical treatment sites and multiple US states, with a minimum monthly quota of 20 O2Vent devices to be delivered to patients, per site.
“We are thrilled to have received FDA clearance, which enables us to sell the O2Vent® Optima in the US – a core market for Oventus alongside Canada and Australia,” says CEO of Oventus, Chris Hart, M.Phil, BSc, BDSc, says in a statement.
“We look forward to officially launching our material agreements within US sleep channels and working with our customers across their various treatment sites to deliver Optima devices to patients. With key regulatory clearances now in place and a robust cash balance following our recent capital raising, we are in a strong position to scale sales substantially across our key markets,” says Hart.




