Dental Clinicians’ Observations of Combination Therapy in PAP Intolerant Patients
Based on the experiences of four highly experienced dental sleep practices, this pilot study sought to determine whether combination therapy, in which a PAP interface and mandibular advancement splint are physically connected, may provide a more acceptable therapeutic modality in obstructive sleep apnea patients who have abandoned conventional PAP therapy.
By Anne E. Sanders, MS, PhD, MS; Martin A. Denbar, DDS, DABDSM; John White, DDS, DABDSM; Ronald
S. Prehn, ThM, DDS, DABDSM; Robert R. Rogers III, DMD, DABDSM; Thomas Pardue, BA; Max Schweizer,
BS; and Greg K. Essick, DDS, PhD
Patients with obstructive sleep apnea (OSA) attribute their poor tolerance of positive airway pressure (PAP) therapy to the presence and fit of headgear and chin straps, claustrophobia, unstable or inadequate fit of mask to facial contours, air leakage, mask-related skin ulceration and headache, aerophagia, and uncomfortably high pressure levels. As a result, only about 50% of patients continue to adhere with therapy after 1 year.
Anecdotally, sleep clinicians claim better tolerance when PAP therapy is used in conjunction with a mandibular advancement splint (MAS), but published evidence is scarce. The MAS stabilizes and advances the mandible, simulating jaw thrust and clearing the upper airway for unobstructed breathing. Improved tolerance of this combination therapy (CT) is attributed to fewer pressure-related complaints. El-Sohl et al showed in 10 patients that CT reduced both the effective continuous PAP and pressure-related complaints shortly after implementation. Consistent with lower effective pressures, Borel et al found that velopharyngeal resistance is lower with CT than with a nasal mask alone. In both studies, the MAS and PAP mask were not physically connected.
The PAP interface, however, can be physically connected to and supported by a MAS, eliminating the need for headgear. One early case report described a patient whose responses to PAP and to MAS therapy were poor, but who achieved near normal sleep respiration (AHI <7 events/hr) and a lower minimally effective PAP upon stabilization of the mandible in an upward and forward position, as corroborated elsewhere. By analyzing existing data from four dental sleep practices, we sought to determine whether PAP delivered through an interface connected to a MAS can offer a more accepted therapeutic alternative for OSA patients who have failed conventional PAP therapy.
METHODS The study employed a retrospective cohort design in which four dental sleep medicine experts in separate US practices retrieved existing data from records for all consecutive patients aged ≥18 years meeting inclusion criteria. The inclusion criteria were: physician diagnosis of OSA and referral to a dentist; having failed conventional PAP therapy; having attempted MAS-PAP within a specified time interval; and being on active recall for therapy evaluation. Clinicians were diplomates of the American Board of Dental Sleep Medicine with collective experience treating more than 14,000 patients with oral appliances for sleep-disordered breathing over a total of 79 years. The Biomedical IRB at the University of North Carolina at Chapel Hill approved the study.
De-identified diagnostic and CPAP titration information from patients’ sleep study reports and clinical records of CT were extracted and recorded on a standardized pretested electronic form.
Investiation was restricted to a single commercially available CT device – the TAP-PAP Chairside (manufactured by Airway Management Inc). In this device, a horizontal rod is secured to the upper component of a MAS, and a nasal pillow/hose assembly is attached using an acrylic material.
Tolerance was defined by clinician report that the patient was continuing to use PAP/MAS, based on periodic recall and ongoing evaluation by the dental sleep expert in the dental office. Acceptability was evaluated by patient report of complaints. Evidence of efficacy was determined in a subset of patients by comparison of follow-up polysomnogram or home sleep study against the diagnostic polysomnogram.
Data were imported into StataCorp LP Stata 13.1 Statistical Software for statistical analysis. Pearson chi-square tested the significance of differences between categorical variables. A paired t-test tested the null hypothesis that apnea-hypopnea index (AHI) values at OSA diagnosis did not differ from those obtained following PAP/MAS.
RESULTS Among 92 OSA patients aged 25 to 85 years (mean 55 years), the mean polysomnogram-determined diagnostic AHI value was 37.6 (standard deviation (sd): 25.9). Half (n=46) met the diagnostic threshold for severe OSA (AHI ≥30), a quarter (n=23) had moderate OSA (AHI 15–29.9), and the remainder (n=23) had mild OSA (AHI 5–14.9). Because the proportion of PAP/MAS tolerant patients did not differ between the practices (P = 0.612), results report pooled data. Overall, 65 patients (70.7%) tolerated PAP/MAS therapy,having used it for 14.0 months on average (sd: 11.1). Although tolerance was higher in patients with severe OSA (76.1%) than mild OSA (69.8%), this difference was not statistically significant (P = 0.421).
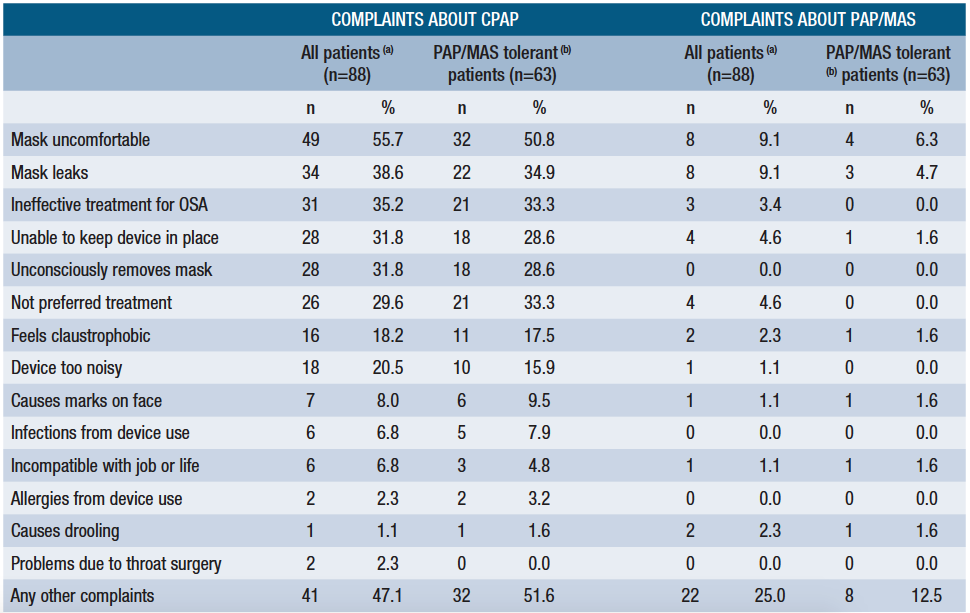
Patients expressed substantially fewer complaints to their dentists about PAP/MAS than conventional PAP (Table 1). The most common complaints about conventional PAP related to mask discomfort and leakage, and the perceived ineffectiveness of PAP. Again, mask discomfort and leakage were the most common complaints about PAP/MAS, but these were reported by only a small minority of patients.
A follow-up polysomnogram or home sleep test was available for 34 of the 65 PAP/MAS tolerant patients while using PAP/MAS. Patients with severe OSA were more likely to have had the follow-up polysomnogram than those with mild OSA (P = 0.023). In these patients, AHI values decreased from an untreated mean of 48.0 (sd: 28.3) events/hour to a treated mean of 3.1 events/hour (sd: 3.8) (P < 0.001), indicating that, on average, PAP/MAS eliminated OSA.
DISCUSSION In this study of patients who had abandoned conventional PAP, 70.7% were reported using PAP/MAS for 14.0 months, on average, after initiation of treatment. PAP/MAS tolerant patients expressed to their sleep dentists substantially fewer complaints about the CT than conventional PAP. CT reduced the AHI to less than 5, on average, achieving values not dissimilar from those achieved with conventional PAP.
Several explanations may account for better tolerance of CT. Borel et al found that joint use of PAP with MAS was at least equally effective as nasal PAP alone by increasing airway patency beyond that achieved solely with PAP. Using a phrenic nerve magnetic stimulation paradigm, the investigators showed greater decrease in velopharyngeal resistance with CT. Since resistance values determined this way are related to pressures at which the pharynx collapses, the joint therapies act synergistically to improve velopharyngeal area patency, the area primarily implicated in upper airway collapse.
El-Sohl et al1 found that the CPAP intolerance was associated with high pressure-related complaints. In agreement with greater pharyngeal patency demonstrated by Borel et al, El-Sohl et al also demonstrated that CT lowered the optimal pressure 29% on average and was well tolerated.
PAP therapy adherence is influenced by the mask interface. Typically, when a nasal mask results in air leakage via the mouth, an oronasal mask is tried. However, the higher pressures required for oronasal masks are associated with greater air leakage, more arousals, more residual respiratory events, dry mouth, and worse adherence than nasal masks. In addition, their manner of attachment tends to push the mandible and tongue backward, increasing the airflow resistance.
Combination therapy may be successful with a lesser degree of jaw advancement than required for treatment with a mandibular advancement splint alone, thereby minimizing dental side effects.
(a) Data about complaints missing for 4 patients (b) Data about complaints missing for 2 patients Table 1. Frequency of patient complaints about treatment modality (CPAP and PAP/MAS), in rank order of CPAP complaints for all patientsA nasal interface used with MAD overcomes the aforementioned problems. The effective pressure is typically lower than with a nasal mask. Rather than pushing the mandible backward,it is stabilized in a neutral or forward position, lowering velopharyngeal resistance and improving upper airway patency. As the mouth is kept closed, air leakage and mouth breathing are minimized.
Because nasal pillows have less facial contact, problems of claustrophobia, air leaks around the eyes, and pressure sores are minimized. In their randomized cross-over study, Massie and Hart compared nasal pillows to nasal masks using pressures ranging from 5 to 14 cm H2O. They reported no differences in treatment efficacy, effective CPAP pressure, Epworth sleepiness score, or quality of life measures. The nasal pillows produced fewer adverse effects, less air leakage, less difficulty sleeping and maintaining sleep, and improved sleep quality.
The nasal pillows in our study were supported via a rod extending from the maxillary component of the MAS, obviating need for a chin strap and headgear to support the air interface. This confined facial contact to the external nares and lip vermilion over which the supporting rod extended. Moreover, the rigidity of the connection with the MAS secured the nasal pillows in a stable “locked” relationship with respect to the external nares to minimize air leaks and thus disruptive arousals from sleep. Air leakage is a major deterrent to the use of PAP, hence the substantial reduction in complaints of “air leakage” and “not being able to keep in place” with PAP/MAS may, in part, underlie its success.
PAP/MAS-tolerant patients had remarkably fewer complaints about the CT than conventional PAP. Our expectation that the addition of MAS would cause discomfort in masticatory muscles, TMJ, and teeth, was not met. CT may be successful with a lesser degree of jaw advancement than required for treatment with a MAS alone, thereby minimizing dental side effects. The use of CT as a first-line therapy, rather than a rescue, may decrease the number of patients who remain untreated from nonadherence to PAP therapy. Compared to conventional PAP, CT appears to offer improved comfort and interface stability, and a reduction in air leakage.
We recognize the many limitations of this pilot study. Data were limited to existing clinical records of community-based dental sleep practices. Because of this, the patients were not matched either within or across the four practices. There was no standardization of the PAP titrations, types of masks employed, or counseling that the patients received on use of conventional PAP or combination therapy. The amount of jaw advancement with PAP/MAS was not standardized within or between practices, which could have affected tolerance of the CT. There was no standardization of the follow-up PSG or home sleep study, and these data were not available for approximately half the CT-tolerant subjects. Data were not available for the nightly duration of PAP/MAS use. Nonetheless, the consistency of the experiences reported across the four unrelated, expert dental sleep practices supports the general conclusion that PAP/MAS can provide an effective treatment for OSA in many otherwise CPAP-intolerant patients and merits formal evaluation in a prospective controlled clinical trial. SR
Anne E. Sanders, MS, PhD, MS, epidemiologist, is assistant professor in the School of Dentistry at the University of North Carolina at Chapel Hill. Interested in the effects of sleep-disordered breathing on oral health, she—along with Greg Essick and colleagues—found that adults with symptoms of OSA were at greater risk of developing first-onset temporomandibular disorder than adults without these symptoms. She also reported an association between sleep-disordered breathing and chronic periodontitis among 12,469 participants in the Hispanic Community Health Study/Study of Latinos, who participated in an overnight home sleep test and a periodontal examination.
Martin A. Denbar, DDS, DABDSM, is affiliated with Austin Apnea & Snoring Therapy in Austin, Tex. John White, DDS, DABDSM, is affiliated with South Carolina Dental Sleep Center in Greenville, SC. Ronald S. Prehn, ThM, DDS,DABDSM, is affiliated with the Center for Facial Pain and Dental Sleep Medicine in The Woodlands, Tex. Robert R. Rogers III, DMD, DABDSM, is affiliated with Pittsburgh Dental Sleep Medicine in Wexford, Pa. Thomas Pardue,BA, Max Schweizer, BS, and Greg K. Essick, DDS, PhD, are affiliated with the School of Dentistry, University of North Carolina at Chapel Hill. Essick is also affiliated with the Center for Pain Research and Innovation at the University of North Carolina at Chapel Hill. Conflict of interest disclosure: Essick has received materials unrelated to the TAP-PAP (the product described in this study) for teaching and research purposes at the University of North Carolina at no cost or on loan from the company that markets the TAP-PAP, Airway Management Inc. All other authors declare no conflict of interest. CONTACT Corresponding author Dr Greg K. Essick at essick@email.unc.edu and (919) 537-3287.