9 Alternative Therapies for Obstructive Sleep Apnea
CPAP is the gold standard for obstructive sleep apnea, but what about patients who can’t—or won’t—tolerate it or who need an additional therapy? We profile the spectrum of FDA-approved therapies, including when to try them, considerations for each, and new developments.
DEVICES
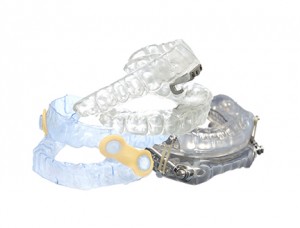
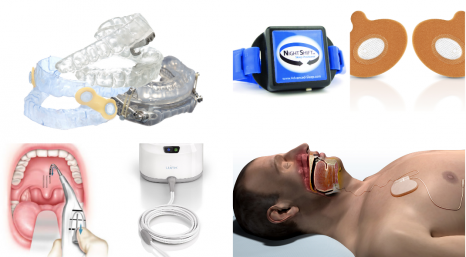
Oral Appliances
Information source: Dr Kathleen Bennett, president, American Academy of Dental Medicine
How it works: Oral appliance therapy (OAT) helps prevent the collapse of the tongue and soft tissues in the back of the throat by supporting the jaw in a forward position, keeping the airway open during sleep.
When to try it: alternative therapy for mild to moderate OSA; after CPAP refusal or failure for all OSA severities; in conjunction with CPAP (may lower CPAP pressure)
Appropriate AHI: Up to 30. For severe OSA patients, only after initial trial of CPAP, oral appliances have been shown to be 50% effective.
Other considerations: Patients with lower BMI and lower AHI have higher success rates with oral appliances. It is most effective for supine dependent OSA. Patients with craniofacial retrognathism—when the lower jaw sits back behind the upper jaw—also have been shown to have success with oral appliances. Patients with steep mandibular planes or long necks have more difficulties reaping the benefits of an oral appliance.
Patients who should NOT try it: those with central sleep apnea or those with morbid obesity; those with poor dentition—it’s best to first restore the patient’s teeth; those with acute TMD derangement or disc displacement resulting in a limited ability to open mouth
New developments in 2014: A study showed objective measurement of OAT compliance is feasible using an embedded microsensor. Results suggest the overall therapeutic effectiveness of OAT may be similar to CPAP.1 Another found that remotely controlled mandibular positioner (RCMP) predicted therapeutic success with significant accuracy, and the predictive protrusive position was effective in 87% of the patients.2 The health outcomes study published by the American Thoracic Society compared the health effects after 1 month of using CPAP versus OAT. It found that health outcomes were similar between the two treatments, suggesting that the greater efficacy of CPAP is offset by the higher compliance with OAT.3
Concerns & Responses:
Concern: There’s no way of knowing if OAT will work on patients before they try it.
Bennett: It is important for sleep physicians to collaborate with a dentist who can conduct a thorough intraoral examination to assess candidacy for an oral appliance. The dentist will evaluate the patient’s teeth, jaw, and airway, determine the protrusive range with a measuring device, and review the data of the sleep study in order to help determine the chance of success.
Concern: Most oral appliances don’t objectively monitor compliance.
Bennett: Objective compliance measurement for OAT is a recent development in the field, and it will become increasingly common in the years ahead. However, it should be noted that the Vanderveken study published in Thoraxfound no significant differences between objective and self-reported oral appliance compliance. Therefore, until objective compliance monitoring becomes standard, self-reports from patients can provide useful clinical insight.
Concern: Oral appliances don’t generally improve AHI as much as CPAP does.
Bennett: While CPAP has a higher efficacy, low patient compliance decreases its overall effectiveness. Because of its higher compliance rate, OAT can produce similar outcomes as CPAP. Neither treatment is perfect, but both options offer positive benefits. It is important to tailor the treatment to the individual needs of each patient to promote long-term therapeutic success.
More information: Sutherland K, Vanderveken O, Cistulli P. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10(2):215-227.
Night Shift
Information source: Dan Levendowski, president, and Philip R. Westbrook, MD, chief medical officer, Advanced Brain Monitoring
How it works: Worn on the back of the neck, Night Shift (by Advanced Brain Monitoring) begins to vibrate when users begin to sleep on their back and slowly increases in intensity until a position change occurs.
When to try it: first line therapy for positional OSA; in combination with CPAP (may lower CPAP pressure), oral appliance therapy, surgery, or Provent to improve outcomes
Appropriate AHI: positional mild, moderate, or severe OSA when nonsupine AHI <20
Other considerations: While positional therapy can reduce the overall AHI to the nonsupine severity, its effect on snoring is more variable. For patients with an elevated supine apnea index, overall snoring may increase with positional therapy because supine apneas are replaced with snoring. Also, to avoid long-term complications from side sleeping, patients should select a pillow(s) that align the head with the spine during lateral sleep and/or allow the cervical and thoracic spine to be as horizontal as possible during prone sleep.
Patients who should NOT try it: those with acute neck, shoulder, or back pain, those with cardiac arrhythmia corrected with an artificial pacemaker, or who have skin sensitivity or an open wound around the neck.
New developments in 2014: Advanced Brain Monitoring received FDA clearance for Night Shift in June 2014. A study appeared in the Journal of Clinical Sleep Medicine in August 2014. A second study showing the capabilities of Night Shift as a screening device accepted for publication. Welltrinsic Network members can get discounts for their patients. Night Shift is listed on FSS fee schedule so VA Centers can purchase it at a discount for their patients.
Concerns & Responses:
Concern: I can advise the patient to sleep on two tennis balls in a sock to stay off his back for a much cheaper price.
Westbrook: Studies suggest that both efficacy and long-term compliance are poor with the tennis ball approach, plus the tennis ball approach does not provide compliance or effectiveness monitoring.
Concern: Night Shift is reactive. It doesn’t keep the patient off his back to begin with.
Westbrook: This is actually an advantage. Night Shift provides the patient 15 minutes before initiating position avoidance feedback to enable those who feel it is necessary to initiate sleep on his/her back. This delay can be reduced to 0 or increased to 30 minutes depending on patient preferences.
Concern: Night Shift will wake my patient up due to the vibration, thereby still interrupting his sleep.
Westbrook: Night Shift decreases the number of arousals in patients with POSA. In our study, the number of arousals due to sleep disorder breathing exceeded the number of supine attempts resulting in positional feedback by a factor of 15. Use of Night Shift resulted in significant reductions in stage N1 and increased in N2, while REM and sleep efficiency increase and arousals and awakenings were significantly reduced.
More information: Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10(8):863-71.
Winx Sleep Therapy System
Information source: David P. White, MD, chief scientific officer, ApniCure
How it works: Winx (by ApniCure) generates negative pressure in the oral cavity, which draws the soft palate and uvula forward, and stabilizes the tongue position, thus enlarging the upper airway.
When to try it: after refusal or failure of CPAP; first line therapy for patients who choose Winx when offered all options
Appropriate AHI: all OSA severities OK but probably most commonly effective in those with AHI between 10 and 50
Other considerations: best for patients who prefer to be able to easily change positions during sleep; have a BMI <40 kg/m2; can breathe easily through the nose
Patients who should NOT try it: Patients who have a severe respiratory disorder, such as severe lung disease, like COPD; those with loose teeth or advanced periodontal disease; anyone under the age of 18. Winx should not be used to treat central sleep apnea.
New developments in 2014: A new version of the Winx Mouthpiece—with a softer, more flexible posterior bar that sits over the back of the tongue—was released in the summer and increases comfort. A clinical study for a different mouthpiece design that improves the response rate with Winx to >63% of subjects was also completed.4 The nightstand console unit has also been improved and is now almost completely silent.
Concerns & Responses:
Concern: There’s no way of knowing if Winx will work on patients before they try it.
White: A single night sleep study either in the lab or in the home can easily determine if the Winx device will work in a given patient. The demonstrated efficacy or lack of efficacy on the first night has been shown to be a durable effect over time.
Concern: Most payors won’t cover Winx.
White: That is true at this time. However, insurance coverage will increase over time and many patients are willing to pay for Winx out of pocket as they will get a full refund of the console only over the first 30 days of use if Winx is ineffective or not well tolerated. ApniCure also has a customer service team that will attempt to gain coverage from the patient’s insurance.
Concern: Patients won’t want to empty a saliva canister in the morning.
White: The majority of Winx users report that the system is easy to use, clean, and maintain. Most patients only generate about 5-6 mL of saliva per night, which can easily be washed from the canister each morning with a little water.
More information: Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med. 2013;14:830-837.
Provent Sleep Apnea Therapy
Information source: Matt Williams, president, Theravent Inc
How it works: During inhalation, the proprietary microvalve technology in Provent (by Theravent) opens, allowing the user to breathe in freely. When exhaling, the valve closes and air passing through the nose is directed through two small air channels. This increases the pressure in the airway (expiratory positive airway pressure or EPAP), maintains pressure, and helps to keep the airway open until the start of the next inhalation.
When to try it: most commonly used after refusal or failure of CPAP; can be used as a first line therapy; can also be successful when combined with either positional or chin strap therapy
Appropriate AHI: appropriate for all OSA severities
Other considerations: The ideal patients are newly diagnosed mild/moderate OSA patients without significant comorbidities, and CPAP compliant patients looking for alternatives for travel, though many other patients are viable candidates for Provent.
Patients who should NOT try it: those with severe breathing disorder (including respiratory muscle weakness, bullous lung disease, bypassed upper airway, pneumothorax, pneumomediastinum, etc); those with severe heart disease (including heart failure); those with pathologically low blood pressure; those with an acute upper respiratory (including nasal, sinus, or middle ear) inflammation or infection or perforation of the ear drum
New developments in 2014: The FDA approved a lower resistance version of EPAP, Theravent, which is an over-the-counter product indicated only for primary snoring. Theravent LITE generates approximately 1/10th the pressure of Provent, and Theravent Max generates approximately half the resistance of Provent.
Concerns & Responses:
Concern: Provent can’t objectively measure compliance.
Williams: The Provent Nasal Cannula is a diagnostic tool to be used with the Provent Sleep Apnea Therapy device during sleep studies, enabling practitioners to confirm product efficacy. The cannula allows transmission of pressure signals from the nose to a pressure transducer of a physiologic recorder with a standard interface designed to attach the nasal cannula to standard pressure transducers, and a custom connection to the Provent Therapy device.
Concern: There’s no way of knowing if Provent will work on patients before they try it.
Williams: This is similar to any medication; the only way for a patient to know if a lipid lowering medication or an asthma treatment works is to use the therapy for a week or up to a month, then be reassessed. To confirm CPAP works for a patient, an overnight diagnostic is used to confirm efficacy. The same can be done with Provent.
Concern: Most payors won’t cover Provent.
Williams: Physicians and patients need to voice their request for Provent to be a covered benefit. At this time, the insurance companies have told us they don’t hear a need from physicians and patients for coverage.
More information: Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34(4):479-485.
IN-OFFICE PROCEDURES
Pillar Procedure
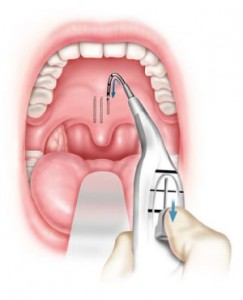
Information source: Craig Schwimmer, MD, MPH, FACS, chairman and chief medical officer, Pillar Palatal, LLC
How it works: Stiffens the soft palate, decreasing its flutter and stabilizing the retro-palatal airway. This is accomplished in a nondestructive way by the placement of small woven inserts into the soft palate, under local anesthetic.
When to try it: after refusal or failure of CPAP; first line therapy for interested patients who meet criteria
Appropriate AHI: mild to moderate OSA
Other considerations: It does not require the removal or destruction of tissue, and is therefore less painful and less risky than traditional palatal stiffening procedures. For these reasons, the Pillar Procedure may be indicated in any patient with a significant palatal component. Because OSA typically does not result solely from palatal flutter, results are optimized when the Pillar Procedure is combined with other anatomically appropriate treatments.
Patients who should NOT try it: those without a significant palatal component to their snoring or OSA, those with known allergy to the implant material, or those with inadequate palatal length
New developments in 2014: Schwimmer acquired the Pillar Procedure from Medtronic Inc in early 2014.
Concerns & Responses:
Concern: Risk of extrusion and infection.
Schwimmer: Over 45,000 Pillar Procedures have been performed. Not a single “serious” complication has been reported. The overall reported rate of extrusion is less than 1%. The reported rate of infection is less than 1%. Pillar has been on the market in the United States for over 12 years, and has been shown to be a remarkably safe procedure.
Concern: AHI doesn’t significantly improve for most patients.
Schwimmer: Multiple studies, including two meta-analyses, demonstrate reliable, meaningful improvement in AHI–as well as other outcome measures such as Epworth Sleepiness Scale, O2 saturation, and QOL scores.
Concern: Pillar Procedure alone may not adequately treat a patient’s OSA.
Schwimmer: That is absolutely true. But the soft palate is a significant contributing factor in most patients with OSA, and by combining the Pillar Procedure with other minimally invasive treatments, many patients can achieve significant improvement, without the pain, risk, and inconvenience of surgical intervention.
More information: Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Otolaryngology-HNS. 2013;123(1):269-76.
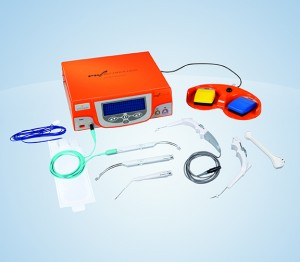
Somnoplasty
Information source: Kamaal Jarrett, associate product manager, ENT, Olympus America Inc
How it works: Somnoplasty, also known as temperature-controlled radio frequency (TCRF), is a minimally invasive surgical technology that uses radiofrequency current to reduce tissue volume in a precise, targeted manner. (Olympus makes a somnoplasty device.)
When to try it: after refusal or failure of CPAP; first line therapy for patients who choose TCRF when offered all options
Appropriate AHI: mild to moderate OSA
Other considerations: none
Patients who should NOT try it: There are no known absolute contraindications to the use of RF surgery. The use of somnoplasty probes and G3 RF workstation is contraindicated when—in the judgment of the physician—electrosurgical procedures are contrary to the best interest of the patient.
New developments in 2014: none
Concerns & Responses:
Concern: There is a risk of bleeding and infection.
Concern: It reduces snoring but not sleep apnea.
(Responses not provided.)
More information: Powell NB, Riley RW, Troell RJ, Blumen MB, Guilleminault C. Radiofrequency volumetric reduction of the tongue. A porcine pilot study for the treatment of obstructive sleep apnea syndrome. Chest.1997;111(5):1348-55.
SURGERIES
Uvulopalatopharyngoplasty (UPPP) with or without Tonsillectomy
Information source: Kathleen L. Yaremchuk, MD, department chair, Otolaryngology Head and Neck Surgery, Henry Ford Hospital, Detroit
How it works: The most common type of oropharyngeal surgery for OSA, UPPP, enlarges the retropalatal upper airway by excising a portion of the posterior soft palate and uvula with trimming and reorientation of the tonsillar pillars. The tonsils, if present, may be excised as well.
When to try it: after refusal or failure of CPAP
Appropriate AHI: N/A (AHI is not a success predictor)
Other considerations: Anatomy is the main predictor of success. Favorable anatomy for UPPP includes large tonsils and favorable tongue placement (small base of tongue).
Patients who should NOT try it: Some argue that patients with a BMI of 40 and above should opt for bariatric surgery over oropharyngeal surgery. But Yaremchuk has had success with UPPP in patients with BMIs in this range and so characterizes high BMI as a “relative” contraindication.
New developments in 2014: More and more surgeons are opting to perform a drug-induced sleep endoscopy (DISE) prior to oropharyngeal surgery in order to find out exactly where the blockage is and help determine the responders versus the nonresponders. Yaremchuk does it immediately preceding the surgery.
Concerns & Responses:
Concern: Infection risk
Yaremchuk: Infection is very uncommon—it’s probably the least likely problem you’ll experience with this surgery.
Concern: AHI may not significantly improve.
Yaremchuk: AHI is not the only indicator of significant OSA improvement. Success may be better determined by another marker such as the oxygen desaturation index or an inflammatory indicator that is more indicative of sleep apnea’s risks. Also, surgery is not a magic bullet for sleep apnea. But when patients reject or can’t tolerate CPAP, surgery is a viable option. A huge problem is when patients reject CPAP, they feel they have nothing left to try.
*Additional oropharyngeal surgery options: Surgical intervention needs to be tailored to where in the upper airway the obstruction is and what the cause is, Yaremchuk says. Another option is transoral robotic surgery (TORS). TORS excises all or part of the lingual tonsils. And a third option is maxillary mandibular advancement (MMA), in which the upper and lower jaws are surgically moved forward.
More information: Friedman M, Ibrahim H, Lee G, Joseph NJ. Combined uvulopalatopharyngoplasty and radiofrequency tongue base reduction for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2003;129(6):611-21.
Bariatric Surgery
Information source: John Morton, MD, MPH, FACS, FASMBS, president-elect, American Society for Metabolic and Bariatric Surgery (ASMBS), and chief, Bariatric and Minimally Invasive Surgery, Stanford School of Medicine, Stanford, Calif
How it works: Gastric banding, sleeve gastrectomy, and gastric bypass surgery lead to significant weight loss, including lessening the buildup of fat tissue in the upper thorax and neck. Studies have shown that weight loss may result in improvements to OSA.
When to try it: first line therapy for patients with BMIs greater than 40
Appropriate AHI: 15 and above
Other considerations: BMI and AHI are the main considerations
Patients who should NOT try it: Patients with severe pulmonary hypertension need a more extensive plan to be prepared for surgery.
New developments in 2014: Procedures have become very standardized and safe. The effectiveness has become more consistent with the advent of understanding how the procedures work and with pre- and post-surgery education.
Concerns & Responses:
Concern: Most payors won’t cover bariatric surgery.
Morton: Bariatric surgery is routinely covered by major insurers. If it’s not covered, you should make an inquiry as to why it is not. It’s safe and effective for patients in need.
Concern: AHI may not significantly improve.
Morton: There are many studies that have demonstrated AHI reductions. Additionally, bariatric surgery and subsequent weight loss provide better treatment options for patients if they are in need of further treatment. For instance, it can improve CPAP adherence.
*Additional weight loss-related options: Medical weight loss without surgery is also sometimes used, including diet, exercise, behavioral therapy, and pharmacotherapy. Morton says he frequently uses nonsurgical and surgical weight loss options together. Weight loss without surgery is much less significant than surgical weight loss, and the medications have contraindications as well.
More information: Aguiar IC, et al. Obstructive sleep apnea and pulmonary function in patients with severe obesity before and after bariatric surgery: a randomized clinical trial. Multidiscip Respir Med. 2014;9(1):43.
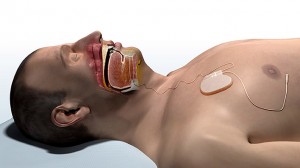
Inspire Upper Airway Stimulation (UAS)
Information source: Inspire Medical Systems
How it works: Inspire therapy is an implanted system that senses breathing patterns and delivers mild stimulation to key airway muscles, which keeps the airway open during sleep.
When to try it: after CPAP refusal or failure
Appropriate AHI: 20 to 65
Other considerations: Inspire UAS may be used in adult patients 22 years of age and older who have been confirmed to fail or cannot tolerate CPAP and who do not have a complete concentric collapse at the soft palate level.
Patients who should NOT try it: those with central + mixed apneas >25% of the total apnea–hypopnea index (AHI); any anatomical finding that would compromise the performance of upper airway stimulation, such as the presence of complete concentric collapse of the soft palate; those who will require magnetic resonance imaging (MRI)
New developments in 2014: In January, results from the STAR trial were published in the New England Journal of Medicine. On April 30, the FDA approved Inspire UAS.
Concerns & Responses:
Concern: Infection risk
Inspire: All surgeries carry a small risk of infection. In contrast to other surgical options to treat sleep apnea, Inspire therapy does not require removing or permanently altering an OSA patient’s facial or airway anatomy. As such, the procedure is less invasive and should result in a shorter recovery time. It also does not require a mask or oral appliance. The serious adverse event rate in the STAR trial was <2%.
Concern: It’s uncertain what the long-term side effects are of continuously stimulating the hypoglossal nerve.
Inspire: There have been no reported long-term side effects from stimulating the hypoglossal nerve.
More information: Strollo PJ, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139-149.
Sree Roy is editor of Sleep Review. CONTACT sroy@allied360.com.
REFERENCES
1. Vanderveken OM, et al. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91-6.
2. Remmers J, et al. Remotely controlled mandibular protrusion during sleep predicts therapeutic success with oral appliances in patients with obstructive sleep apnea. Sleep. 2013;36(10):1517-25.
3. Phillips CL, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879-87.
4. Emsellem HA, Winslow DH, Siegel LC, Bogan RK, McCullough PA, Stiles J. Safety and effectiveness of oral pressure therapy with a new oral interface. SLEEP 2014; 37(abstr suppl):A146(4017).